Phosphorus
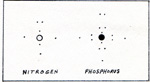
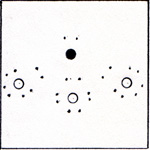
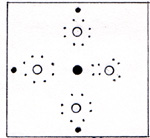
The similarity between nitrogen and phosphorus is due to the 5 outer electrons which they both have. The total number of electrons is 7 for nitrogen, and 15 for phosphorus. The two inner electrons of nitrogen take up very little space. The space occupied by the 10 inner electrons of phosphorus make a big difference. (Fig. 1) To view an enlarged figure, click on the existing figure. To return to text, click Back. |
|
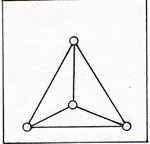
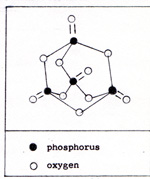
The outer electrons of phosphorus cannot form strong triple bonds, because the distance between the electrons and the atomic nuclei is greater in phosphorus than in nitrogen. The outer layer is roomier in phosphorus than in nitrogen. Therefore the paired electrons can easily separate. The phosphorus atom often forms compounds in which phosphorus is bonded to five other atoms. Nitrogen is limited to bonding with three other atoms. Because the phosphorus atoms are not triple bonded, phosphorus reacts with oxygen spontaneously. Uncombined phosphorus is never found. It has to be produced in the laboratory or the chemical factory. White phosphorus is produced in an electric furnace from a mixtue of calcium phosphate, Ca3 (PO4)2 , silica, SiO2 , and carbon, C : 2Ca3 (PO4)2 + 6SiO2 + 10C -----> P4 + 6CaSiO3 + 10CO The product is P4. (Fig. 2) |
|
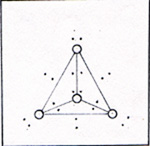
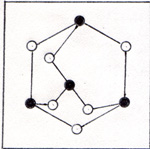
Each phosphorus atom is attached to three other phosphorus atoms. The phosphorus comes out of the furnace in a gaseous mixture of phosphorus molecules, P4 and carbon monoxide molecules, CO. The calcium silicate remains in the furnace as a liquid, and is periodically drained off. The points of zero force for the outer electrons of the phosphorus atoms are shown in figure 3. |
|
The electron pattern is superimposed on the three-dimensional diagram of figure 2. Each pair of electrons is farther away from a nucleus than would be the case if four atoms were lined up. (Fig. 4) |
|
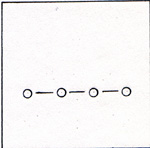
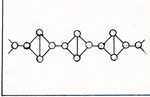
The electrons in figure 3 are farther from the nuclei, because the electrons of other atoms are nearby and repel them. The bonds in figure 2 are weak compared with the bonds in figure 4. A bond in figure 2 has a strength of 1.88 x 10-12 erg. You might expect the phosphorus atoms to line up and have stronger bonds. If I limit the molecule to P4, there are only three bonds per molecule. The tetrahedrally arranged P4 molecule has 6 bonds. 3 x 3.47 x 10-12 erg = 10.41 x 10-12 erg 6 x 1.88 x 10-12 erg = 11.28 x 10-12 erg Once a P4 molecule is formed, a fifth phosphorus atom can't interfere with it. The four atoms assemble one by one. Phosphorus atoms have to find each other in a gas that has 10 carbon monoxide molecules to 1 phosphorus molecule. Four atoms have time to get into various shapes before a fifth atom comes along. If the fifth atom arrives before the P4 tetrahedron forms, there is a good chance that the next interaction with another molecule will break an atom off the chain. After that the four remaining atoms get another chance to form a tetrahedron. It is much harder to tear an atom away from a tetrahedron, because each atom in the tetrahedron has three bonds. The hot gases that issue from the furnace in the phosphorus refining process are disharged under water. The P4 condenses and becomes a soft solid. The CO bubbles out as a gas. Carbon monoxide is a useful product as a fuel and as a reactant in the production of several compounds. I can heat white phosphorus in the absence of oxygen at 700 K for several hours, and get red phosphorus. (Fig. 5) |
|
Starting with white phosphorus, I no longer have the problem of carbon monoxide getting in the way. The heat breaks one of the bonds of the P4 molecule. This relieves the crowding of the electrons of all of the other bonds. The bonds become stronger. Neighboring F4 molecules become bonded to each other through the electrons of the bonds that were broken. Red phosphorus is more stable than white phosphorus, because it has stronger bonds. There is a more stable form of phosphorus than red phosphorus, black phosphorus. If I heat white phosphorus under high pressure in the absence of oxygen, I get black phosphorus. (Fig. 6) |
|
White phosphorus is stored under water to keep it away from oxygen. I take a small piece of white phosphorus and expose it to air. I darken the room and I can see the phosphorus glow. The glow comes from the reaction of some of the phosphorus with some oxygen. The reaction is spontaneous, but it is slow. The glow comes from the increased vibration of the parts of the molecules. The oxygen molecule accelerates toward the phosphorus atoms. When the oxygen atom gets close to the electrons of the phosphorus atoms, all points of zero force shift. This causes all of the atoms to vibrate. Some of the vibrating parts oscillate with frequencies in the range of visible light. The oscillations of electrons in molecules are more complicated than the oscillations in free atoms, because each electron is influenced by the fields of other electrons in the same atom. In any case the principle still holds, as it does in the hydrogen atom, that a photon is emitted when the frequency of the oscillation of the electron is the same as the resonant frequency of the oscillation of the photon. |
|
In an oxygen molecule, the point of zero force for one of the electrons is determined by all of the forces in the molecule. The points of zero force and the strength of the forces keep shifting and altering, but there is just enough stability for the electron to emit an occasional photon. Under ordinary circumstances, at room temperature, the amplitude of oscillation of any electron in an oxygen molecule is not enough to produce a photon in the visible range. The encounter between an oxygen molecule and a piece of white phosphorus is no ordinary circumstance. Each P4 molecule bristles with electrons. The P4 is in solid form. This presents a surface which bristles with electrons. Oxygen is attracted very strongly to electrons. The oxygen molecule accelerates toward the surface of white phosphorus. During the acceleration and the rebound, the oxygen molecule has a higher kinetic energy than average, therefore a higher average temperature. As a result, the vibrations of the phosphorus nuclei increase, stretching the bond between them, At the same time, the proximity of the electrons of the phosphorus weakens the bonds. A few oxygen molecules break apart, and some phosphorus molecules react with some oxygen atoms. The piece of white phosphorus can sit exposed to the air for about 20 minutes before it bursts into flame. During the incubating period, the temperature of the surface gradually rises. At the moment of ignition, the temperature at one spot is at the kindling point. The product of the reaction of phosphorus and oxygen is phosphorus pentoxide. (Fig. 7) |
|
The P4O10 that forms is built on a skeleton of P4. Only with P4 as a starting material, is it possible to get P4O10. Red phosphorus and black phosphorus produce other oxides of phosphorus. Another indication that the oxygen reacts with the P4 without disturbing the P4 skeleton, is the product of the burning of white phosphorus with insufficient oxygen. (Fig. 8) |
|
This is P4O6. The oxygen atoms have single bonds to two phosphorus atoms. As one of the oxygen atoms approaches the P4 molecule, it is attracted by the pair of electrons of one of the P-P bonds. (Fig. 9) |
|
Two bonds are formed with the oxygen atom and the two phosphorus atoms. (Fig. 10) |
|
When phosphorus is burned in air, where the oxygen supply is ample, the product is P4O10. There are four more oxygen atoms on the product molecule. |
|
Figure 11 shows a phosphorus atom in P4O6 before the arrival of the extra oxygen. (Fig.11) |
|
There is an unbonded pair of electrons. The next oxygen atom has four paired outer electrons and two unpaired outer electrons. (Fig. 12) |
|
The oxygen atom uses its unpaired electrons to form a double bond with the phosphorus atom. (Fig. 13) |
|
When the oxygen supply is inadequate, only single bonds form. That shows that two single bonds yield more energy than one double bond on P4O10. The product P4O10, called phosphorus pentoxide, is a gas at first. As the molecules find each other, they aggregate as dust particles. In time, the dust collects as a powder. It is very useful as a drying agent. Once it absorbs water, the pentoxide molecule dissociates into phosphoric acids. A typical phosphoric acid is H3PO4. (Fig. 14) |
|
One of the oxygen atoms is not bonded to a hydrogen atom, and it has a double bond to the phosphorus atom. Matches that can be struck on any surface are made of white phosphorus. White phosphorus is highly reactive because of the weakness of the bonds in P4. The bonds are weak because each electron is too close to several other electrons. The white phosphorus matches have a protective coating to keep oxygen away until friction removes some of the protection. Safety matches are made with sulfur on the match, and red phosphorus on the striking surface. Red phosphorus can burn, but it has to be coaxed. The bonds in red phosphorus are slightly stronger, because the electrons are somewhat separated. Black phosphorus is inactive because it has stronger bonds. There is a compound of hydrogen and phosophorus that is somewhat analogous to ammonia, the compound of hydrogen and nitrogen. The phosphorus compound is phosphine, PH3. Ammonia is NH3. Both compounds are gases that are fairly stable in air, but can burn. It is possible to prepare an explosive mixture of phosphine in air. Ammonia is highly soluble in water. Phosphine is only slightly soluble in water. One of the minerals found in rocks is hydroxyapatite, Ca5(PO4)3OH. The enamel of our teeth is 90% hydroxyapatite. Our bones are 23% hydroxyapatite. The ion PO43- is called phosphate. It is an important component of DNA, the molecule that carries the genetic material that controls the growth and development of living things. It is also part of ATP, the compound that transfers energy in all cells of the body, especially muscle cells. The use of a material for something as important as life, makes us wonder whether the supply can be exhausted. Before there were living things on earth, the phosphate was in the rocks. The apatites, and some of the other phosphates which are in rocks, are insoluble in water. Some rain water contains acid. Apatites are soluble in acid. Weathering and erosion make some of the phosphate available to living things. Some living things extract phosphates directly from rocks. Lichens secrete acid, and slowly dissolve the rock. Plants that have roots in soil, also produce acid, and free some of the phosphate in that way. Animals get phosphates from plants, and return phosphate to the soil in their wastes and dead bodies. There seems to be a complete cycle with continued supply of phosphate assured. When streams carry phosphate to a lake, there is a burst of growth of living things. This is noticeable as a bloom among the algae. It indicates that the algae, up to that point, were existing within the limit of their phosphate supply. A fresh supply of phosphate made them reproduce and grow suddenly. It can happen that a lake will contain great amounts of phosphate, and the large quantities of algae will settle on the bottom of the lake. The lake can become a bog, and, eventually, dry land. The phosphate will be buried with the algae. This will not cause a shortage of phosphate, because rocks containing phosphate will still abound. Streams carry phosphate to the ocean. Some of the phosphate is used by living things. We get that phosphate back by consuming sea food. Birds that eat sea food return to the land some of the phosphate in the form of wastes. On the other hand, some of the phosphates in the ocean encounter calcium ions. The calcium ions and the phosphate ions combine to form insoluble compounds, which precipitate on the ocean floor. Some of that phosphate will remain in the ocean bed until geologic changes raise the ocean floor and make it dry land. So far, the phosphate cycle seems to work well except for what happens to lakes. There are similar cycles for carbon and nitrogen. Both of these elements are essential to living things. These cycles seem to be working fairly well. The algae were well supplied with carbon and nitrogen so they could bloom when supplied with extra phosphate. Nevertheless, scientists must always be on guard for signs of failure of cycles. A possible source of trouble may be acid rain. Other problems may arise entirely unforseen. |
|
|
| |
|
|
||
| previous page |
|
next page |
|
|
||
|
|
||