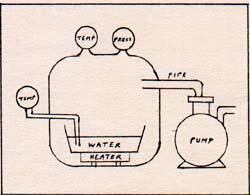
 The container is a vacuum chamber . There are two thermometers and one pressure gauge. There is a water pan on an electric heater, with a remote control. There is a vacuum pump with an automatic pressure control.
The container is a vacuum chamber . There are two thermometers and one pressure gauge. There is a water pan on an electric heater, with a remote control. There is a vacuum pump with an automatic pressure control.
Water
The pursuit of scientific knowledge can begin in a glass of water. The study of water can bring us far. Later we can invest in supercolliders and tremendous telescopes. Living bodies contain more water than anything else. Although water is unique in many respects, it does have features in common with the rest of the world's matter. In learning about water, we learn some things about everything else. Protons join neutrons and become nuclei. Electrons join nuclei and form atoms. Molecules can be free, as gas, or they can combine as liquid or solid. Water is liquid at room temperature. Ice melts at 273K. K is short for Kelvin. Zero K is the absolute zero of temperature. Zero centigrade is 273 K. The boiling point of water is 373 K. These figures are valid only at standard atmospheric pressure at sea level. In a closed space where the air pressure is one thirty-third as great as standard atmospheric pressure, water boils at 300 K. When the air pressure is higher than standard, ice melts at lower temperatures than 273 K. So we see that, in order to understand water, we must know something about the atmosphere. The air is 78 percent nitrogen and 20 percent oxygen. The rest of the air is a mixture of gases like carbon dioxide, water, and argon. The temperature in the room can be the same for any number of molecules. It merely is required that the average kinetic energy per molecule remain the same. The number of molecules per unit volume determines the air pressure. If we have a glass of water at room temperature, the water evaporates and empties the glass in the long run. Other things being equal, if we reduce the air pressure, the rate of evaporation goes up. We must examine the mechanism of evaporation. At room temperatue, the molecules of matter in the liquid state are in motion constantly. Sometimes a fast-moving molecule strikes a slow-moving molecule. In the encounter, some of the motion of one molecule is added to the other molecule. On occasions, two succesive encounters could increase the speed of one molecule at a time when it is at or near the surface. If it is moving upward, it leaves the water. The molecules of the surface attract the fast molecule and reduce its speed. Quantitatively, the amount of kinetic energy that the molecule loses becomes potential energy. That means that, when the molecule returns to the surface of water at that point, it will return to the speed it had on departure, this time in the reverse direction. Its kinetic energy is restored when its potential energy is gone. In the case where the leaving molecule does not return, it accumulates potential energy but retains enough kinetic energy to keep moving. The total of the potential energy plus the remaining kinetic energy is equal to the original energy that the molecule had when it left the surface of the water. To get back to the atmospheric pressure, reducing the pressure means reducing the number of air molecules per square centimeter facing the surface of the water. If a water molecule on the way out, collides with an air molecule, one of the results could be a forced return to the water. When the air pressure is reduced, there are fewer collisions. Nothing is simple. If we add a draft blowing air across the surface of the water, the escaping water molecule may collide with an air molecule that pushes it horizontally. It gains Kinetic energy and a better chance to escape. In the meantime, back at the glass of water, the fastest moving molecules have escaped, reducing the average speed per molecule, reducing the temperature. A point of equilibrium is reached at which the number of water molecules leaving is equal to the number of water molecules returning. Anyway the water that sits around in the glass for some time is at a lower temperature than the air in the room, because the process entails the loss of the fastest moving water molecules. There is a cute demonstration, in which a glass flask is half-filled with water and heated to a lively boil. The heat is turned off and a stopper promptly inserted at the mouth of the flask. The flask is then inverted. Water is sprinkled over the flask to cool the remaining air inside the flask. That reduces its pressure, and the water begins to boil violently. I want the reader to notice that I have used the word energy more frequently in this essay than in others. It is very convenient that way. If I had to revert to real causes all the time , the essay would be more lengthy and monotonous. That which comes easily may not be worth much. There is the danger that energy, which is only a convenient concept, might be mistaken for a real thing. Let the reader beware. The speed of the average air molecule at room temperature is 5 x 104 cm/sec. Pretend that I have one air molecle in a room, and that the size and shape of the room is such that the average trip across the room in any direction is 300 cm. The molecule makes 167 trips per second. 5 x 104 cm/sec divided by 3oo cm = 167 per second The single molecule fills the room. It is the equivalent of 167 molecules making one trip per second. The temperature in the room can be the same whether there is one molecule in the room or billions of molecules. It merely is required that the average kinetic energy per molecule remain the same. The pressure depends on the number of molecules in the room, the temperature, and the volume of the room. It is generally easier to find the pressure by measurement with a barometer or a pressure gauge than to calculate it. A cube 29 x 29 x 29 cm3 has a volume of about 24.4 liters. If the cube contains 6.022 x 1023 molecules of air at 298 K, it has a pressure against its walls of 1.01 x 106 dyne per cm2. If I remove exactly half of the molecules, I reduce the pressure by exactly half. If I want to know the pressure of a single molecule, I divide the pressure by 6.022 x 1023. I find that the pressure is 1.68 x 10-18 dyne per cm2 per molecule of air at 298 K in a cube of 29 cm x 29 cm x 29 cm. The pressure of a single molecule is much less if the room is larger. If I double the volume, I cut the pressure in half. I perform an experiment using some expensive equipment. (Fig.1) |
 The container is a vacuum chamber . There are two thermometers and one pressure gauge. There is a water pan on an electric heater, with a remote control. There is a vacuum pump with an automatic pressure control.
The container is a vacuum chamber . There are two thermometers and one pressure gauge. There is a water pan on an electric heater, with a remote control. There is a vacuum pump with an automatic pressure control.
|
I can set the vacuum pump control at any desired pressure. The pump runs only until that pressure is reached. If the pressure rises a little, the pump automatically starts up again until the pressure is correct. I start with a setting of 9.2 torr. Torr is the name for the unit of pressure that used to be called the millimeter of mercury. A pressure of 1 atmosphere is a pressure of 760 torr. A pressure of 9.2 torr is equal to about 1/83 atmosphere. As the pressure goes down from 760 torr to lower pressures, the temperature of the water decreases somewhat. Even the temperature of the air goes down. The vacuum pump removes molecules from the vacuum chamber. With fewer molecules of air above the surface of the pan of water, the water evaporates. Water evaporates by the departure from its surface of the fastest moving H2O molecules. The molecules of H2O move farther if they are not interfered with too soon by other molecules. Reduced pressure means fewer molecules per unit volume. At a particular temperature, there is a definite average number of molecules at the surface with a sufficient kinetic energy to escape from the liquid and not return. The departure of these molecules reduces the temperature of the water. A molecule leaves the water with maximum translational energy. As the molecule moves away from the surface of the water, it decelerates because of the attraction of the surface of the water. If the molecule has less than escape velocity, it returns to the surface with all of its kinetic energy restored. If the molecule has exactly the escape velocity, it decelerates to a standstill, having a potential energy in the surface-molecule system equal to the kinetic energy that it formerly had. If the molecule leaves with more than escape velocity, it still has kinetic energy as well as potential energy. If it weren't for interference from surrounding molecules, all of the H2O molecules with escape velocity, or higher velocity, would not return to the water's surface. The number of molecules per unit volume in the chamber is the measure of interference. Departing molecules reduce the temperature of the water. Returning molecules raise the temperature. When the number of returning molecules equals the number of departing molecules, there is no net evaporation and no change in temperature. The water loses temperature by losing the fastest-moving molecules, lowering the average kinetic energy. The missing kinetic energy is in the form of potential energy of the water surface-H2O molecule system. When the vacuum pump reduces the pressure to 9.2 torr, the temperature is reduced to 283 K on both thermometers, the one in the water, and the one in the air. The pump maintains a constant pressure of 9.2 torr. There is no net evaporation, and the temperature remains a constant 283 K. I turn the heater control to a setting of 284 K. The water boils, but the temperature of the water and the air remain constant at 283 K. Any excess of molecules is removed from the chamber by the vacuum pump. I turn up the heater control, but this only increases the rate of boiling, without changing the temperature from 283 K. I change the setting of the vacuum pump control to 23.8 torr. The pumping stops and the boiling stops. The temperature of the water rises to a new setting. The boiling stops because the extra H2O molecules add to the total number of air molecules in the chamber, and the pressure rises. H2O molecules return to the water at the same rate as they leave. There is no net evaporation once the thermometer reads the same temperature as the heater control setting. I set the heater control at 298 K. As the temperature of the water rises, the pressure gauge shows an increase until 23.8 torr is reached. The pressure results from the addition of H2O molecules in the air of the chamber by evaporation. The temperature and pressure settle at 298 K and 23.8 torr, and there is no boiling. I set the temperature control at 300 K. The water boils, but the thermometer stays at 298 K. I change the setting of the vacuum pump control to 760 torr. The boiling stops. I turn the heater control to 373 K. The pressure rises as the temperature rises, because evaporation adds molecules to the chamber. At 373 K and 760 torr, there is no action. I raise the temperature setting to 373.5 K. The water boils. If I raise the temperature control to much higher settings, I only increase the rate of boiling without raising the temperature. The temperature does not rise, because the heat goes into producing more of the fastest-moving molecules. The fastest molecules escape, carrying the heat with them. When the temperature control remains at 373 K, and the pressure control is at 760 torr, there is no boiling, and no net evaporation, because the number of H2O molecules returning equals the number of H2O molecules leaving the surface. That is clear in an atmosphere of steam, at 373 K, but it is not obvious when the surrounding atmosphee is air at 298 K. Picture a cubic centimeter of steam side by side with a cubic centimeter of air, H2O on the left, air on the right. Each cm3 has 1.97 x 1019 molecules. After the collision, the H2O molecule may be moving in any direction. Half of the possible directions bring the H2O molecule, more or less, to the right, and half to the left. Therefore, a batch of H2O molecules that start off toward the right, have half of them heading toward the left after the first collision. Half of the half that are moving toward the right, are moving leftward after the second collision. After a dozen collisions, a batch of 4100 molecules is reduced to one molecule moving toward the right consistently. All the other molecules retreat part of the time. The complete mixing of two cubes of molecules takes a long time. Steam does not penetrate the air readily. The atmosphere in contact with the surface of water is more like steam than like air. The air surrounding the steam functions as an automatic pump that is always adjusted to atmospheric pressure. That is why as many molecules of H2O return to the water as leave, when the temperature of the water is kept constant at 373 K, but is not boiling. When water boils, more H2O molecules leave than return. In order to maintain the boiling, heat is added. The heat goes into the kinetic energy of the fastest-moving molecules. The average molecule among the fastest moving molecules leaves the surface of the water with a kinetic energy of 7.514 x 10-13 erg. Compare the 7.514 x 10-13 erg, the energy required to break away a molecule from the surface, with 7.1 x 10-12 erg, the energy required to separate two hydrogen atoms. The latter is about nine times the former. The force that binds the H2O molecule to the surface of liquid H2O is worth investigating. A water molecule has an oxygen nucleus in the center, two electrons close to the nucleus, eight electrons in the outer layer, and two protons beyond the outer layer. (Fig. 2) |
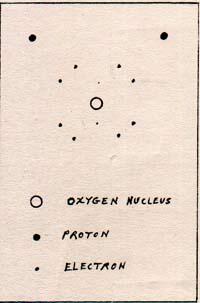
The diagram does not do justice to the water molecule. It needs to be in three dimensions. Take a ping pong ball and place pairs of dots at four locations, as widely spaced as possible. That is a much better picture.
|
Another way to draw the water molecule is as a figure with four points and three dimensions. The points are equally distant from each other. (Fig. 3) |
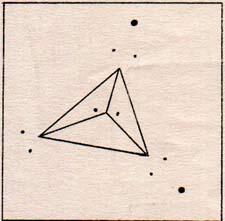
A real water molecule has its two unaccompanied electron pairs somewhat more widely separated than the two electron pairs that have protons for company. Two pairs of plain electrons repel each other more than two pairs of electrons accompanied by protons. The presence of a proton lessens the negativity of the group.
|
There is a positive side and a negative side to a water molecule. (Fig. 4) |
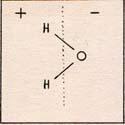 In the diagram, the negative side is to the right of the dotted line, and the positive side is to the left . In a drop of water, the molecules orient themselves with the proton of one molecule close to the oxygen of the other molecule. Each water molecule becomes somewhat attached to four others.
In the diagram, the negative side is to the right of the dotted line, and the positive side is to the left . In a drop of water, the molecules orient themselves with the proton of one molecule close to the oxygen of the other molecule. Each water molecule becomes somewhat attached to four others.
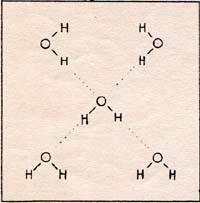 This needs a three-dimensional model. The array can be as big as an iceberg. When water is solid, in the form of ice, the array is crystalized. It keeps its shape. When the water is liquid, each molecule is capable of leaving its position. Nevertheless, there is a sort of tansitory bond in the water.
This needs a three-dimensional model. The array can be as big as an iceberg. When water is solid, in the form of ice, the array is crystalized. It keeps its shape. When the water is liquid, each molecule is capable of leaving its position. Nevertheless, there is a sort of tansitory bond in the water.
|
The heat of vaporization is the quantity of energy that it takes to pull the molecule of water away from the attraction of four other water molecules. The vibration of an oxygen atom in the environment of four protons that surround it also increases the space between water molecules. On the average, the attraction between molecules is weakened at higher temperatures, because of the increased spacing. In evaporation, the H2O molecule has to get away from four molecules. The lower the temperature of the water, the closer the water molecules are, the stronger their attraction, the harder it is to break away. Therefore the heat of vaporization is greater at a lower temperature than at a higher temperature. The heat that goes into raising the temperature of the water from 298 K to 328 K helps the eventual separation of H2O from the surface. People perspire in order to keep the body temperature from rising. More H2O molecules evaporate than return, when the air is sufficiently dry and warm. When the relative humidity is 100%, H2O molecules return as frequently as they leave. There is no net evaporation and no cooling. Actually, there is a little evaporation, because the skin temperature is slightly higher than the air temperature, when the air temperature is lower than 100 degrees fahrenheit. In chilly weather, H2O molecules that collide in the air form groups of two or more, sometimes thousands. People don't sweat in cold weather. The associated H2O molecules in the cool, moist air, settle on the skin. Body heat supplies the heat of vaporization that separates the H2O molecules. High humidity makes people feel hotter in the summer and cooler in the winter. Melting ice is similar to boiling water. In both cases there is a large quantity of potential energy. In both cases there is no change of temperature while two states, liquid and gas, or sold and liquid, exist closely interacting. The array of H2O molecules in an ice crystal is hard to illustrate on a flat page. A group of six molecules, in three dimensions, might look like (Fig. 6) |
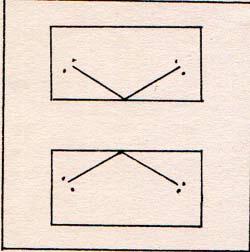
 This shows the shape, if you can visualize the three dimensions. It shows only two connections per molecule. These connections are called hydrogen bonds. They are much weaker than the usual bonds. The two hydrogens for molecule 1 that are shown, are to molecule 2 and to molecule 6. The two hydrogen bonds to molecule 3 that are shown, are to molecule 2 and to molecule 4, etc. Each of the molecules, 1 to 6, has two additional hydrogen bonds, which are not shown.
This shows the shape, if you can visualize the three dimensions. It shows only two connections per molecule. These connections are called hydrogen bonds. They are much weaker than the usual bonds. The two hydrogens for molecule 1 that are shown, are to molecule 2 and to molecule 6. The two hydrogen bonds to molecule 3 that are shown, are to molecule 2 and to molecule 4, etc. Each of the molecules, 1 to 6, has two additional hydrogen bonds, which are not shown.
|
The shape of the array is caused by the way the electrons are distributed in the water molecules. The pairs of electrons are separated as widely as possible. The hydrogen bonds form along the lines connecting electron pairs on neighboring molecules. You can get a picture of the structure of one molecule by using two sheets of paper. Draw the same angle on each sheet, approximately 110 degrees. (Fig. 7) |
|
Hold up one sheet with the vertex up, and the horizontal edge lined up north and south. Hold the second sheet with the vertex down, and resting on the vertex of the first sheet. The horizontal edge of the second sheet is lined up east and west. (Fig. 8) |
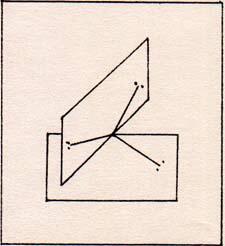
Fig. 9
In order to simplify my presentation, I use a ficticious array. (Fig. 9) |
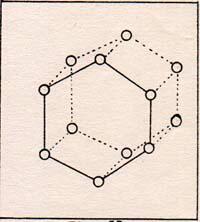 The molecules are in their positions of zero potential energy. This is ice at zero K. Ice at 200 K is just like ice at 100 K, except that the amplitude of vibration is greater. Even so, the molecules do not get far from their zero points.
The molecules are in their positions of zero potential energy. This is ice at zero K. Ice at 200 K is just like ice at 100 K, except that the amplitude of vibration is greater. Even so, the molecules do not get far from their zero points.
|
At 200 K, some of the H2O molecules get exceptionally high temperatures. They get as far as the center of the space in the array of Fig. 9 . In the trip from the point of zero force to the middle of the figure, the molecule loses kinetic energy, and gains potential energy. If the molecule returns to its zero point, it regains its kinetic energy. If the molecule reaches the middle of the array, and has kinetic energy left over, it continues to move away from its zero point. It is a peculiarity of the array of ice molecules that there is sufficient empty space for free water molecules to wander through. At temperatures below 273 K the wandering molecules find a vacancy in the array and come to rest again. At 273 K and 760 torr, the average molecule that can break away has enough kinetic energy left over ,when it has its potential energy, to have a temperature of 273 K. The net effect for this molecule is that it has received exceptional energy, which it has converted into potential energy, and the molecule still has its temperature of 273 K. At 273 K, the melting point of ice, any heat that a piece of ice receives from the environment goes into increasing the potential energy of exceptional molecules without raising the temperature of the ice. When all of the molecules of a sample have potential energy equal to the energy that carries a molecule from the array to the middle of a space, the sample is entirely liquid. Any additional heat received by the liquid raises its temperature. Ice melts at a temperature lower than 273 K when the pressure is higher than 760 torr. The pressure pushes molecules toward the center of the array, speeding the melting process. When the surroundings are at a lower temperature than 273 K, water molecules lose their potential energy, and move into their points of zero force. The approximate angle of between 105 degrees and 110 degrees between hydrogen bonds dictates the six-sidedness of the array. Look at a snowflake through a lens. It has six-sided geometry. The heat that is converted from kinetic energy to potential energy in the melting process is 9.97 x 10-14 erg per molecule of ice. The heat of vaporization of water at 373 K and 760 torr is 6.74 x 10-13 Water is not the only substance that has heats of vaporization and melting, but it is the prime example. All crystals have a heat of melting. All liquids have a heat of vaporization. Substances that do not crystalize behave as very cool liquids at low temperatures. They flow very slowly or so imperceptively that they seem to be quite solid. Some substances that are crystaline solids at room temperature dissolve in water. The heat of melting is supplied by the potential energy that exists between parts of the crystals and parts of the water. The attraction between water and some crystals is so strong, that water vapor is extracted from the air to become part of the crystals. When water is poured over some crystals, the temperature rises fast. |
|
|
| |
|
|
||
| previous page |
|
next page |
|
|
||