Physical Chemistry
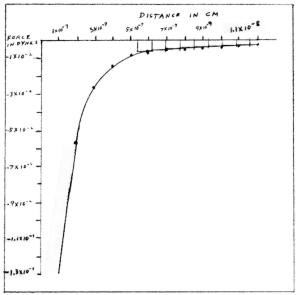
According to standard science, an electron (as part of an atom) can receive only certain exact quantities of energy. When the energy arrives as a photon, the electron is promoted to a higher energy level. If that photon does not have an exactly acceptable quantity of energy, it is not absorbed. The photon with the unacceptable frequency, is reflected, or transmitted, or scattered, but not absorbed. Collisions with exactly the right quantities of energy can promote an electron to higher energy levels. Collisions with incorrect quantities of energy cannot excite an electron. These rules also apply to other particles than electrons. Photons that excite oscillations between atoms in a compound, or between nuclei in a crystal, are usually infrared. Here too, only those photons with energies that match permitted energy levels can be absorbed. Gamma ray photons can excite the interior of an atomic nucleus. This is distinct from oscillation between nuclei in a crystal, in which the entire nuclei move as units. Excitation by gamma rays causes the individual protons and neutrons within the nucleus to be promoted to higher energy levels. Internal nuclear excitation makes the nucleus radioactive. It is unstable until it emits a gamma ray, or some other particle that carries off excess energy in the proper quantity. An ordinary molecule or crystal, when excited, can return to the ground state by emitting a photon with the proper infrared frequency, or by a collision of the proper magnitude. An electron which has been promoted, or excited, can emit a photon of the proper frequency. The electron falls to a lower energy level in the process. An alternative is to lose the proper quantity all at once in a collision of the proper magnitude. This also brings the electron to a lower level, the ground state. When an electron is two levels higher than its ground state, it can lose the proper energy to drop either one level or two levels. If it drops one level, it can hesitate a short time before it drops again to the ground state. Hesitations of this kind are usually in the time range between 10-8 and 10-6 seconds. The potential energy of the proton-electron system is due entirely to the attractive force between the proton and the electron. Standard science does not have the short -range repulsive force of the neg-pos theory. When the electron is in the ground state, there remains potential energy for the remaining distance, and for the rapidly increasing attractive force. This residual potential energy, of unknown quantity, is inaccessible and in forbidden territory. If there were no energy levels, and no short-range repulsive force, the force between the proton and the electron in a hydrogen atom would have values that are calculated from coulomb's law: F = q1 x q2 divided by r2 q1 = q2 = 4.8 x 10-10 esu r is the distance of separation in cm. For example, I find the force at a separation of 2 x 10-10 cm. -(4,8x10-10)2 divided by (2 x 10-10)2 F = -2.304 x 10-19 divided by 4 x 10-20 F = -5.76 F dyn = -5.76 dyn An attractive force is negative. A repulsive force is positive. I calculate the force for several separations. I make a data chart.
I draw rectangles on the graph beteen r = 5.29 x 10-9 cm and r = 1.2 x 10-8 cm. I find the area of each rectangle.
|
|
I add up the areas. The total area is the difference in potential energy between r = 5.29 x 10-9 cm and r = 1.2 x 10-8 cm. The potential energy is -4.36 x 10-11 erg. By a formula for finding the kinetic energy of a particle in circular orbit in a given field, it is calculated that the electron at r = 5.29 x 10-9 cm has a kinetic energy of 2,18 x 19-11 erg. This is the kinetic energy required by quantum theory as the minimum remaining energy at level 1. The equation for the total energy is: Etot = Ek + Ep Substitute the known values in the equation: Etot = 2.18 x 10-11 - 4.36 x 10-11 Etot erg = - 2.18 x 10-11 erg The electron starts out at a distance greater than 1.2 x 10-8 cm with a total energy of zero. As the electron falls to level 1, it loses 2.18 x 10-11 erg to a photon that is emitted, and it converts 2.18 x 10-11 erg to kinetic energy. Having 4.36 x 10-11 erg less potential energy than zero, the electron has a potential energy of -4.36 x 10-11 erg. Having 2,18 x 10-11 erg of kinetic energy at the same time, the electron has a net energy of -2.18 x 10-11 erg, also called the total energy. Compare this with the data in the neg-pos theory:
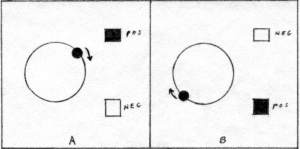
The theories have in common the quantity 2.18 x 10-11 erg as the difference in energy between two states. Only, one is negative and one is positive. A system of two hydrogen atoms is a molecule. The molecular bond is the most probable location of the two electrons. The hydrogen molecule is in the ground state, when the protons have their minimum vibration consistent with the uncertainty principle, and the electrons are in their smallest possible orbital. It is consistent with quantum theory to expect the protons to have enegy levels. Intermediate energies are forbidden. During the vibration of the protons, the molecular orbital stretches and shrinks like a rubber band. This does not change the energy level of the electrons. A change in electron energy levels takes much more energy than a change in energy levels of the protons. If a photon were to have energy equal to the difference in energy levels for a proton, it would have a frequency in the infrared range. No such photon is observed for the hydrogen molecule. However, there is a photon in the visible range, that has the proper frequency to be absorbed by an electron in the molecular orbital of hydrogen. Absorption is observed for a series of photons with slightly higher frequencies also. The standard explanation for the additional frequencies is that they represent the energy levels of the protons, superposed on the energy of the electron. There is no infrared radiation to show energy levels in the hydrogen molecule, because the hydrogen molecule is symmetrical in its distribution of positive and negative charge. The molecule does not have a positive side and a negative side. Therefore it does not radiate or absorb photons through the vibration of its protons. This is also true of other molecules, which have two atoms of a single element, for instance, oxygen,nitrogen, and chlorine. There are experimental data for the radiation and absorption of infrared photons by hydrogen chloride molecules. The chlorine nucleus has 35 times the mass of the hydrogen nucleus. Therefore, practically all of the vibrating is done by the hydrogen proton. The negative charge of the molecule is closer to the chlorine than to the proton. The proton oscillates toward and away from the chlorine atom. The proton can have all intermediate energies through transfer of energies by collision. Only photons with matching frequencies and energies are emitted or absorbed. Neg-pos theory applies just as well to the vibration of the proton, as to the oscillation of the electron. A rotating hydrogen chloride molecule can emit or absorb photons, because, relative to a photon, the positive of a proton is alternately above and below the center of negative charge of the chlorine atom, when the rotation is in the same plane as the photon. (Fig. 3) |
|
When the proton rotation is in resonance with the arriving photon, the proton is accelerated downward in step A, and upward in step B. The accelerating proton gains neg-pos, and increases its rate of rotation. However the rotation does not increase at the same rate as the rotational energy, because part of the rotational energy is potential energy. As the molecule rotates faster, the proton moves away from the chlorine atom. Since the force between the proton and the chlorine atom grows at an uneven rate, complicated by various long-range and short-range forces, some attractive, some repulsive, the potential energy grows unevenly. Therefore, the energy of the proton does not always coincide with the frequency of rotation, and is in resonance with the photon only at certain frquencies. In neg-pos theory, the molecule may transfer rotational energy in any intermediate quantity by collision. The molecule may transfer energy to or from a photon only at resonant frequencies. In quantum theory, the molecule is forbidden to rotate at any intermediate rates. The molecule can receive or transfer energy only in discrete quantities. In quantum theory, energy levels are assigned wherever there is an opportunity. A typical energy level diagram for vibrational and rotational energies appears in (Fig. 4) |

|
Long lines are vibrational levels. Short lines are rotational levels. Although some of standard chemistry deals with the submicroscopic level of atoms and molecules, most of it deals with bulk matter. For large quantities, the units like erg and esu are uncomfortably small. Textbooks give bond energies in kilocalories per mole, or joules per mole. The kilocalorie is the Calorie that is referred to in nutrition. The nutrition Calorie is equal to one thousand of the calories of physics. One calorie, according to physics, is the amount of heat that raises the temperature of one gram of water one degree centigrade. A mole of molecules is 6.022 x 1023 molecules. A mole of atoms is 6.022 x 1023 atoms. The mole, as a unit of multitude, is designed to match the gram, as a unit of mass. One mole of carbon atoms has a mass of 12 grams. Carbon 12 is the standard. When two moles of hydrogen atoms combine into one mole of hydrogen molecules, they transfer 103 kilocalories (kcal for short) to the surroundings. The bond energy of hydrogen is 103 kcal per mole. Two moles of oxygen atoms combine into one mole of oxygen molecules, and yield 118 kcal/mole. That is the bond energy of oxygen. Let one mole of hydrogen react with one half mole of oxygen : H2 + O -----> H2O
H-O-H has bond energy 2 x 109.9 kcal = 219.8 kcal 219.8 - 162.0 = 57.8 The energy of the reaction is 57.8 kcal. |
|
57.8 kcal is the amount of energy that is transferred to the surroundings. The O-H bond in H-O-H has a bond energy of 109,9 kcal. The product H2O is a gas. When H2O is in the liquid state, it is held together by forces betwen H2O molecules, in addition to its O-H bonds. Therefore there is an energy of reaction for: H2O vapor ------> H2O liquid The energy of the reaction is 10.5 kcal. That does not change the energy of the H-O bond. It only adds the intermolecular forces to the other forces. The energy of 10.5 kcal/mole is the heat of vaporization of water at 298 K, room temperature. These reactions are carried out in an ordinary laboratory, at room temperature, and at a constant pressure of one atmosphere. The constant atmospheric pressure is specified, because part of the energy of a reaction goes into pushing back some of the air. The heat content of a substance is called enthalpy. The symbol for enthalpy is H. The change in enthalpy is called delta aitch. Absolute enthalpies are not known. Changes in enthalpy are listed in charts in textbooks and handbooks. The energy of a reaction carried out at constant pressure is the delta aitch of the reaction. When the heat content of the products is greater than the heat content of the reactants, delta H is a positive number. A positive delta H indicates the arrival of energy from the surroundings. When the heat content of the products is less than the heat content of the reactants, delta H is a negative number. A negative delta H indicates the loss of energy to the surroundings. The use of negatives leads to confusion. In the accounting, reactants vs. products, the figures are not heat content. They are the figures for bond strength. A molecule with a greater bond strength has given up more energy to the surroundings than a molecule with a smaller bond strength. I have been writing the figure for the energy of the reaction as a positive number to avoid confusion. In standard science, the energy of the reaction is usually negative, because delta H for the reaction is usually negative. For instance, for the reaction: H-H + 1/2 O-O -----> H-O-H (gas) delta H = -57.8 kcal The energy of the reaction is -57.8 kcal. In most cases, the reaction with a negative delta H is a permitted reaction. A reaction can have a positive delta H at one temperature or pressure, and a negative delta H at another temperature or pressure. In exceptional cases, the sign of delta H can be misleading. A negative delta H does not always indicate a permitted reaction. There is another criterion for determining whether a reaction goes or doesn't go. It is the function G. If a reaction has a positive delta G, it is forbidden. If a reaction has a negative delta G, it is permitted. delta G = delta H - T delta S T is temperature, S is entropy |
|
The function G is dependent on the function S, which stands for entropy. Entropy, by itself, should be a sufficient criterion for the possibility of a given reaction. But a reaction is permitted if the entropy of the universe increases as a result of the reaction. The entropy criterion is difficult to apply, because it is hard to evaluate the change in entropy of the surroundings. The equation: ^G = ^H - T^S refers only to the reactants and products, and not to the environment. That is manageable. The symbol ^ stands in for "delta". Without the function G, the criterion is ^H. When ^H is negative, the reaction is permitted (with a few exceptions). The following is an example of a permitted reaction, for which ^H is positive: Ag + 1/2Hg2Cl2 ------> AgCl + Hg silver + mercurous choride ----> siver chloride + mercury The energy of the reaction is 1.28 kcal/mole. However, the energy is transferred from the environment to the reaction. The proper form is to write the 1.28 kcal as +1.28 kcal. Thereby, ^H is positive, and it is something of a surprise. The reactants are both solids. One of the products is a liquid. A liquid is more random than a solid. The liquid state is more probable than the solid state. Therefore, the entropy of liquid mercury is more than the entropy of mercurous chloride. The change in entropy of this reaction is a positive number. When that number is multiplied by 298, the absolute temperature, it comes to more than 1280. The units of entropy are calories per mole per degree. In this reaction, ^H is +1.28 kcal, or 1280 cal. Subtracting the figure for T^S from 1280, one gets a negative number for ^H - T^S. Therefore, ^G is negative. Whenever ^G is negative, the reaction is permitted. ^G supersedes ^H as a criterion of permitted reactions. For a reaction with a large negative or positive ^H, it is sometimes better to use ^G as a criterion for a permitted reaction. Sometimes the functiion G is called the free energy function. The quantity of ^G for a reaction is said to be the maximum energy available for the performance of useful work. Every device for getting useful work out of a chemical reaction, must transfer some heat to the surroundings. The minimum heat which must be wasted in that way, is equal to T^S. Since the total energy of the reaction ^H, ^H - T^S must be the maximum energy that might produce useful work. But ^G = ^H - T^S , so ^G is the free energy of the reaction. Unfortunately, only the ideal engine can get ^G work from the reaction. Real engines get 20% to 60% of the maximum possible work. One way to get useful work from a chemical reaction, is to let the reaction occur in an electric cell. (Fig. 5) |

|
The ^G of the reaction can be measured by a voltmeter which draws little or no current. A cell whch gets its current from the reaction : Zn + CuSO4 -------> ZnSO4 + Cu has a voltage of 1.1 volt, when the temperature is 298 K, and the concentration of zinc sulphate is 1 mole per liter, and the concentration of copper sulfate is 1 mole per liter of solution. A reading of 1.1 volt means an energy of 1.1 electron volt for each electron that travels the length of the wire. The electron volt is a unit of energy. In this instance, it is the free energy per electron. One electron volt equals 1.6 x 10-12 erg. One mole of zinc atoms releases two moles of electrons. Two moles of electrons are 2 x 6.022 x 1023 electrons. Each electron has an energy of 1.1 ev. 2 x 6.022 x 1023 x 1.1 x 1.6 x 10-12 = 21.2 x 1011 erg 1 erg = 2.39 x 10-8 cal 2.39 x 10-8 x 21.2 x 1011 = 50.7 x 103 cal 1 kcal = 103 cal 50.7 x 103 cal = 5o.7 kcal ^G = -50.7 kcal This is an excellent procedure for finding ^G for the reaction: Zn + CuSO4 ------> Cu + ZnSO4 However, it does not tell us how much energy will go into useful work. The work of an electric current is performed by means of an electric motor. A motor that is not attached to a burden does not conduct much current. Such a motor runs idly. The voltage across an idling motor is essentially equal to the full voltage of the electrodes of the cell. An idling motor does no useful work. The nearest thing to an idling motor, is the motor in an electric clock. The clock can run a full year before it consumes a cell. In that instance, the useful work of the reaction in the cell is close to 50.7 kcal. The same motor can move a toy truck. That motor is not idling. The friction of the parts of the truck, and the inertia of the truck, hold back the rotation of the motor somewhat. The motor does not run at its idling speed, when it is held back by the truck. A motor that rotates at a reduced speed, permits a larger current to flow. When a larger current flows in a cell, the solution heats up, and the voltage drops. The motor also heats up. With the voltage lower, each electron has less energy for its passage through the wire. The useful work of the reaction in the cell, is closer to 25 kcal than 50 kcal. ^G is not very good for predicting how much useful work can be done by a reaction. ^G is reliable for predicting whether a reaction will go, but that is seldom needed. The best use of ^G is for determining the point of equilibrium for a reaction. The easiest way to find ^G is by an electric cell. Instead of setting up an experiment every time one needs to find ^G, one finds the ^G listed in a handbook. One such chart lists ^G of formation at 298 K. The formula of a compound is followed by the ^G of the reaction that produces that compound. For example:
|
|
A chemist who wants to try a new reaction, does not find the reaction listed in the chart. The chart can be of service , because reactions can be added algebraically. Suppose one wants to find ^G for the reaction: C2H2 + H2 = C2H4 The chart lists:
The ^G of + 50.000 is for the reaction: 2C + H2 = C2H2 The ^G of + 16.282 is for the reaction: 2C + 2H2 = C2H These values of ^G are called standard molecular free energies of formation at 298 K. Formation means the production of the compound from the elements. Since the values of ^G are positive, these compounds cannot be formed from the elements, except by drastic measures. On the other hand, the reverse reactions, the production of the elements from the compounds, are very simple, and have values of ^G. If the first reaction is reversed, and the second reaction is not reversed, the algebraic sum of the reactions is the one that the chemist is looking for. The reverse reaction has the same ^G as the first reaction, except for the sign. If the forward reaction has a positive^G, the reverse reaction has a negative ^G. For example, the ^G for : C2H2 = 2C + H2 is -50.000 kcal This is the reverse of: 2C + H2 = C2H2 whose ^G is +50.000 kcal |
|
The 2C, which appears on both sides of the equation, cancels. H2 appears twice on the left side, and once on the right side of the equation. One H2 cancels. One H2 remains on the left side. 2C + 2H2 + C2H2 = 2C + H2 + C2H4 H2 + C2H2 = C2H4 ^G = -33.718 kcal The negative ^G shows that the reaction is permitted. The number -33.718 is substituted for the symbol ^G. By this means, the value of k is calculated. k is the equilibrium constant. Whenever a reaction is carried out in such a way that the products and the reactants are not separated from each other, some of the product participates in the reverse reaction. Before equilibrium is reached, the quantity of the product increases. The greater the quantity of the product, the more the reverse reaction takes place. As the quantity of the product increases, the quantity of the reactants decreases. With a diminished quantity of the reactants, the forward reaction is diminished. Since the forward reaction decreases, while the reverse reaction increases, there must come a time when the reverse reaction equals the forward reaction. That is equilibrium. The equilibrium constant is the ratio of the product to the reactants. When there is such a large negative ^G, the value of k is so large, that practically none of the reactants remains when equilibrium is reached. The ^G of formation at 298 K for NH3 is only -3.976 kcal/mole. The equilibrium constant is small. At different tempeatures and pressures, the equilibrium constant is different. In order to get the maximum yield, proper values of temperature and pressure are chosen. But that is not the entire story. The product is continually being removed from the process, to avoid the reverse reaction. |
more on physical chemistry | |
|
|
|
|
|
| |
|
|
||
| previous page |
|
next page |
|
|
||
|
|
||