Photochemistry
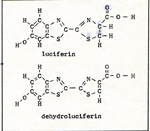
The glowworm has a lot of company. There is a long list of organisms, from bacteria to fish, that produce their own light. Decaying vegetable matter glows by the light produced by fungi. By no means are all of the fungi luminescent, but some microscopic forms and some mushrooms are. In some instances, decaying meat gives off light because of the bacteria that consume the meat. Most bacteria are not luminous; and most decaying meat does not glow. Even some living insects, infected by luminescent bacteria, glow in the dark. Some fireflies are winged as adults. In some species only the males have wings. The light of a glowworm has a function. It helps the male to find a female, and it identifies the species by the rhythm of the flashes. There are some beetles that carry headlights and tail lights, like automobiles. Also among the arthropods are some luminous centipedes and lantern flies. The luminescence of the ocean is caused by single-celled animals. However, the ocean contains many larger animals that give off light. There are jellyfish, sea pens, marine worms, some shellfish, squid, shrimp, brittle stars, and some crabs. In addition, some fish live symbiotically with bacteria that supply them with light. The light which comes from living cells is part of the energy of a chemical reaction. The immediate source of the light is an electron which emits a photon. When such light appears in the flame of a combustion reaction, it is no surprise. The high temperature of the flame keeps enough electrons oscillating with sufficient amplitude so that a noticeable number of electrons emit photons of visible light. In a functioning cell, the temperature is always between the freezing point of water and the boiling point of water. Some cells survive, but don't function, at lower and higher temperatures than those limits. In any case, it is hard to explain how an electron could get such amplitude of vibration in a moderate environment. Even if an electron exists with such vibration in a watery environment, it is more likely to lose its energy by collision than by radiation. Another reason why it is hard to understand this reaction is that it involes an enzyme, ATP, and oxygen. We do know that the main reactant is luciferin, and the main product is dehydroluciferin. (Fig. 1) |
|
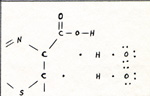
Luciferin and dehydroluciferin are exactly alike except for one pair of hydrogen atoms at the right hand end of the diagram. Many compounds have a pair of hydrogen atoms; yet in some way luciferin is unique. All parts of the molecule contribute to the reactivity of the two hydrogen atoms. Electrons are less attracted to nitrogen atoms than to carbon atoms. Sulfur and oxygen attract electrons strongly. When two atoms with unequal attractions for electrons form a bond, the point of zero force for the electron of the bond is closer to the atom which has the stronger attraction. The enzyme luciferinase is a very large molecule. It has an active site which is just right for holding a molecule of luciferin. The luciferinase also attracts an oxygen molecule and gets the oxygen into the best position to attract both hydrogen atoms of the luciferin. During the departure of the hydrogen atom from the carbon atom next to the nitrogen atom, the second electron of that bond starts to follow the hydrogen atom. While the shifting of the hydrogen atom is taking place, the point of zero force for the second electron is unusually far from the center of the carbon atom. (Fig. 2) |
|
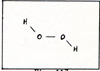
The two hydrogen atoms move off with the oxygen molecule as hydrogen peroxide. (Fig. 3) |
|
With the hydrogen atoms gone, the point of zero force, which was far from the carbon, returns to a location between the two carbon atoms. This gives the electron a long trip back, driven by potential energy comensurate with the distance, and a corresponding store of neg-pos. The electron oscillates with sufficient amplitude to produce a photon with the right resonance frequency. The photon is emitted in the visible range. Another example of chemical luminescence is the red glow in the sky on a clear, moonless night. In the upper air, gas molecules are exposed to all manner of bombardment from outer space. Molecules are cracked apart into atoms. The free atoms combine readily with many other elements. For example, oxygen and nitrogen atoms combine: O + N -----> NO oxygen + nitrogen -----> nitric oxide This may be followed by a second oxidation: O + NO ----> NO2 oxygen + nitric oxide -----> nitrogen dioxide The distance between particles in the stratosphere is so great that two-body collisions can take place far from other particles. In order to form a bond, the oxygen and nitric oxide must yield the energy of the reaction. Air molecules at sea level interact about 109 times a second. Thus the molecules transfer energy without radiating a photon. An electron radiates at an oscillating frequency for about 10-8 seconds before a photon starts to radiate. If the atom which contains the electron is involved in a collision within 10-8 seconds, it loses the neg-pos and the vibration without radiating. In the upper atmosphere, molecules typically go 10-6 seconds between collisions. They get a chance to radiate photons and produce a glow in the sky. |
|
The oxygen atom accelerates toward the nitric oxide molecule. The oxygen atom rebounds. One electron picks up the energy from the vibrating oxygen nucleus. The electron emits a photon, and the nitrogen dioxide molecule becomes stable. It is rid of internal energies of vibration of its parts. The flame of a candle emits photons at sea level even though its molecules collide about 109 times a second. The collisions inside a flame are so violent that they seldom slow electrons below the energy they need to radiate. The electron that retains its strong vibration after collisions emits a photon the first time it gets an interval of 10-8 seconds between collisions. Every object in the room emits photons, when the room temperature is 298 K. These photons are of very low frequencies. Collisions at 298 K, occurring 109 times a second, do not lower the average internal energy of the molecules. Whenever the frequency of the electron and the frequency of the photon are in resonance, uninterrupted for 10-8 secomds, the photon is emitted. This is an infrared photon. The situation at room temperature is the same as the situation at flame temperature. Photons are emitted either way, but the flame can emit high energy photons as well as low energy photons. Photons emitted at room temperature are all of low energy and low frequency. It is not necessary to use an electron to emit an infrared photon. An atomic nucleus in a solid object or in a gas molecule generally oscillates. The frequency of that oscillation is the frequency of the photon that the nucleus emits. When an infrared photon strikes an object, it may be reflected or it may be absorbed, depending on the molecule it strikes. Absorption takes place only when the frequency of the photon is in resonance with some oscillator in the receiving molecule. Some reactions yield light. Other reactions are activated by light. I can mix equal volumes of hydrogen and chlorine gases. As long as I keep the mixture cool and dark, it is almost safe. When I expose the mixture to light, it explodes. Cl-Cl + H-H -----> H-Cl + H-Cl chlorine + hydrogen -----> hydrogen chloride This reaction goes the right way. It does not need any outside energy. As the reaction proceeds, it yields energy to the surroundings. The light kindles the reaction. A spark or a struck match does the same thing. The absorption of one photon of light supplies the energy to break one Cl-Cl bond. A photon of blue light, of frequency 6 x 1014 cycle/sec, has an energy of 4.03 x 10-12 erg, the energy required to break the Cl-Cl bond. The photon is absorbed by an electron. The energy of the absorbed photon makes the electron oscillate in a series of swings past the point of zero force. During the time while the photon is arriving, each new pair of neg-pos adds speed to the swings, until the electron attains escape velocity. The atomic nucleus, which was bonded to the molecule by that electron, promptly joins the escaping electron. A free atom of chlorine attracts hydrogen very strongly. A chlorine atom is capable of displacing a hydrogen atom from a hydrogen molecule. Let a chlorine atom come nearly as close to a hydrogen molecule as the hydrogen atoms are to each other. The chlorine attracts the nearer hydrogen atom about as strongly as the other hydrogen atom does. When the hydrogen protons are vibrating, their motion is occasionally great enough to assist the chlorine atom in its pulling contest. At that point, the bond of the hydrogen molecule is broken by the superior force of the nearer chlorine atom. Hydrogen chloride is formed: Cl + H-H -----> H-Cl + H chlorine atom + hydrogen -----> hydrogen chloride + hydrogen atom A free hydrogen atom can break up a chlorine molecule in a similar manner. H + CL-Cl -----> H-Cl + Cl This constitutes a chain reaction. All of this activity raises the temperature tremendously, and there is an explosion. This reaction goes the right way. A reaction that is driven the wrong way by the energy of photons is: H-I + H-I -----> H-H + I-I hydrogen iodide -----> hydrogen + iodine Here again, a photon is absorbed by an electron in a molecule. The molecule is hydrogen iodide. There is no chain reaction, because the energy of two H-I bonds is slightly greater than the energy of the H-H bond plus the I-I bond. Each H-I molecule has to receive its own private photon to keep the reaction going. The free atoms that are produced, recombine with the first available partner. Sometmes they recombine as H-I. In the long run, all of the H-I is dissociated because it is susceptible to photon attack by visible light. Some of the I-I molecules are broken by photons, but eventually they have no hydrogen atoms with which to react. They must recombine as I-I . Hydrogen is strongly bonded to hydrogen. It takes an ultraviolet photon to break the hydrogen molecule. In spite of the strength of the bond in hydrogen, this is a wrong way reaction, because the sum of the energies of H-H and I-I is less than the sum of the energies of two H-I bonds. |
|
Most chemical bonds have strengths greater than the energy of a visible photon. Photons with higher energies are in the ultraviolet (or higher) range. Ultraviolet photons stimulate chemical reactions in the manner of visible light stimulating the reaction of chlorine with hydrogen. The ultraviolet photons can break many chemical bonds. That makes ultraviolet radiation hazardous to living things. Besides causing unwanted reactions, ultraviolet photons disrupt the genes in living cells. Photons are photons no matter what their frequency. There is no exact boundary between ultraviolet radiation and X-rays. The innermost electrons of the most massive atoms can be expelled by X-ray photons of high frequencies. The remarkable thing about X-rays is their penetration of solids. Photons of visible light can pass through air, water, glass, and some solids. Much can be learned about photons by observing instances of transparency, translucency, and opacity. A photon passes through distilled water at very little risk of being captured. The electrons in water molecules are close to nuclei, where the attractive force is strong. The molecules of water do not absorb photons with frequencies in the visible range, because the electrons in water molecules do not resonate at such frequencies. A photon in the visible range, that arrives at a copper ion,(in a copper sulfate solution) finds an electron that can oscillate at the frequency of the photon, unless it is at a frequency that appears blue to the human eye. The blue photon passes through the copper ion, usually in the original straight line, but sometimes reflected at an angle. When the direction of the photon is changed, it is said to be scattered. The blue color of the copper sulfate solution is due to the non-absorption of blue light, while other colors are absorbed. The copper atom is large. The outer electrons are shielded from the nucleus. The force that restores an outer electron to its point of zero force is weak. The vibration of such an electron involves very little energy. Therefore many photons in the visible range resonate with the outer electrons of copper, and are absorbed. Glass is like water in some respects. The atoms of glass are silicon, oxygen, calcium, and sodium, all small atoms. The electrons in small atoms are close to the nuclei. The forces restoring the electrons to their points of zero force are strong. The electrons resonate at frequencies in the ultraviolet range. Photons of visible light are transmitted, rather than absorbed. Some of the photons are scattered, so that very thick glass is noticeably less than perfectly transparent. There are many absorbers of ultraviolet radiation. Much of sunlight is ultraviolet. Molecules of air, especially ozone, O3, absorb ultraviolet photons. Of the ultraviolet that gets past the shield of the atmosphere, some of it is absorbed by the pigment in human skin, melanin, to protect the rest of the human body. The energy that is absorbed from the ultraviolet photon is used in making vitamin D in the cells of the skin. Ultraviolet photons are absorbed by ordinary glass. There is a glass known as quartz glass, which transmits ultraviolet photons of some frequencies. Most atoms and molecules of any material have some electrons which resonate at the proper ultraviolet frequencies. In small atoms, the innermost electrons respond to ultraviolet. In large atoms, the middle electrons resonate at ultraviolet frequencies. Electrons which resonate at the frequencies of visible light, are dislodged by ultraviolet regardless of frequency. The ultraviolet photon has more than enough energy to give the electron forced oscillation of such amplitude, that it leaves the atom permanently. The excess energy, which is not absorbed by the electron, continues existence as a photon of reduced frequency. The atom remains one electron short. If the lost electron is missing from an inner position, an outer electron gets a new point of zero force, closer to the nucleus. That electron, before it settles down, vibrates at the new frequency with a large amplitude. If it doesn't distribute the excess energy, the electron radiates a photon. The upper atmosphere receives so many ultraviolet photons, and violet photons, that many air molecules have electrons which radiate excess energy. Usually the radiation is in the blue region. This is part of the reason for the blue sky. In addition it appears that blue photons are more susceptible to reflection and scattering, than photons of lower frequency. The blue scatters all over the sky, while the red comes through on its original course, directly from the sun. The light leaves the sun as white light, containing all colors fairly evenly. It arrives looking reddish. |
|
The principles are the same for X-rays as for other photons. The difference is that X-rays are more energetic. They dislodge the innermost electrons of medium size atoms. X-rays of very high frequencies dislodge the innermost electrons of the largest elements. An X-ray photon dislodges an electron, and continues to pass through the medium as a photon of slightly lower frequency, in some cases. For example, X-rays go through cardboard nearly undiminished. They go through aluminum foil slightly diminished. X-rays are absorbed by sheet steel. This can be overcome by using X-rays of still higher frequencies. Most X-rays are absorbed by lead. At frequencies so high, that X-rays and gamma rays are indistinguishable, photons penetrate every element, including lead. Since the intensity of the radiation, after passing through a medium, depends on how many obstacles are in the path, a denser medium absorbs more photons than a less dense medium. An X-ray picture shows that more photons get through glands than through muscles, and more photons get through muscles than through bones. Free atoms, at high temperatures, radiate at certain frequencies. Each element has its own characteristic set of frequencies. Usually only two or three colors are intense, and they are enough to identify the element. The colors can be separated by passing the light through a glass prism . A spectrometer or a spectroscope is an instrument built around a glass prism. In this instrument, the colors appear as separate lines that fall in exact positions on a scale. The scale can be marked in frequencies. The green line of one element is slightly different from the green line of another element. Since the lines fall on different places, they can be distinguished by their frequencies. An atom which permits a particuar frequency, also absorbs that same frequency. Although this is observed in a spectroscope, the atom, or a large group of such atoms, does not have any color when viewed without the spectroscope. The light by an atom is a tiny fraction of the incident light. In the spectroscope, sunlight that passes through a sample of free atoms looks like a rainbow, crossed by a few black lines.The black lines take the place of the absorbed frequencies. The element can be identified by the position of the black lines. Most elements are not available as free atoms. The points of zero force for all of the electrons of an atom are displaced when the atom is part of a molecule. Many electrons in a molecule resonate at visible frequencies. Instead of a few lines, the electrons absorb wide bands of frequencies. Light reflected from molecules often shows a color. It is the color of the part of the sunlight that is not absorbed by the molecule. In general the molecules of larger atoms have more coloring . For example, chlorine is pale green, bromine is dark red, iodine gas is violet, solid iodine is black. Iodine dissolved in alcohol is brown. Iodine dissolved in benzene is pink. Iodine reacts with starch to form a blue compound. |
|
Before the development of sophisticated instruments, which analyze and identify chemicals with little or no help from the technician, color was of some help to the chemist in the task of chemical analysis. Records were kept of the colors of many substances. Here are a few examples:
Observe that these compounds contain heavy metals. The outer electrons of large atoms are loosely held and can oscillate at low frequencies. When the metals are not combined with other elements, they have a metalic luster due to their free electrons. Metals reflect light so well, that there is hardly any opportunity for light to get through the surface of the metal to be absorbed. Among organic compounds are the azo dyes. For example, there are chrysamine G (yellow), para red, Chicago blue, methyl orange, azobenzene (orange red). Each of these compounds has a pair of double bonded nitrogen atoms. The double bonded nitrogen atom holds its electrons very weakly. |
|
|
| |
|
|
||
| previous page |
|
next page |
|
|
||
|
|
||