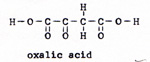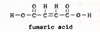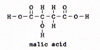Living Motion
The equation for a reaction is usually written with the reactants first and the products last. For example: H-H + Br-Br ------> H-Br + H-Br I can write the equation for the reverse reaction: H-Br + H-Br ------> H-H + Br-Br I do an accounting of bond strengths: |
|
12.16 x 10-12 erg - 10.35 x 10-12 erg = 1.81 x 10-12 erg The bond energies of the reactants are greater than the bond energies of the products by 1.81 x 10-12 erg. I don't expect to see hydrogen bromide decompose into hydrogen and bromine. I refer to such reactions as wrong direction reactions, because the bond energies of the reactants are greater than the bond energies of the products. The reaction in which HBr is the product, is a right direction reaction. The bond energies of the products are greater than the bond energies of the reactants. Hydrogen gas and bromine gas at 332 K, in contact with platinum, combine as hydrogen bromide gas. Suppose I have a supply of hydrogen bromide, and I want to get some bromine liquid. I dissolve hydrogen bromide in water. I bubble chlorine gas into the water. This reaction produces hydrogen chloride and liquid bromine. The water is warmed by the reaction. If necessary, I warm the water still more to cause the bromine to evaporate and become separated from the water. I collect the bromine gas and let it condense into liquid bromine at room temperature. Incidentally, I take care not to come into personal contact with any of these chemicals. They damage living tissue. The reaction that I have just described is: H-Br + H-Br + Cl-Cl -----> H-Cl + H-Cl + Br-Br
|
|
17.50 x 10-12 erg - 16.19 x 10-12 erg = 1.31 x 10-12 erg This reaction goes in the right direction. It is the sum of two reactions:
The H-H cancels because it appears on both sides of the equation. The reaction that produces H-Cl is a right direction reaction: H-H + Cl-Cl ------> H-Cl + H-Cl
14.30 x 10-12 erg - 11.18 x 10-12 erg = 3.12 x 10-12 erg The energy of the reaction for the combined reaction is the difference between the energy of the H-Cl reaction and the energy of the H-Br reaction: 3.12 x 10-12 erg - 1.81 x 10-12 erg = 1.31 x 10-12 erg I use a right direction reaction to drive a wrong direction reaction, when the right direction reaction has an energy of the reaction which is greater than the energy of the reaction which goes in the wrong direction. These are called linked reactions. |
|
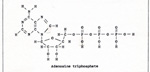
Linked reactions are very common in living things. In photosynthesis, many linked reactions employ the compound ATP, adenosine triphosphate. (Fig. 1) |
|
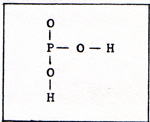
In cells that can contract or move, the energy for the motion always is transferred by means of linked reactions with ATP. The molecule contains a chain of three phosphates. The bond that joins the last phosphorus atom to the rest of the molecule is a very weak bond. That makes a phosphate available. (Fig. 2) |
|
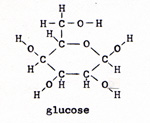
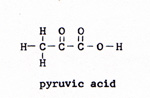
The linked reaction involves the combining of phosphate with some other molecule to form a strong bond. The energy of the reaction drives the wrong direction reaction that the organism needs. It would not take long to exhaust all of the ATP. To replenish the supply of ATP, there is another linked reaction. A glucose molecule is reacted with oxygen. The energy of the reaction is ample to link several wrong direction reactions to the glucose-oxygen reaction. The wrong direction reactions break the strong bonds between phosphate and some other molecule, and reconstruct ATP, with its weak phosphate bond. The ATP with one phosphate missing is ADP, adenosine diphosphate. In the linked reaction with glucose, ADP is joined with phosphate to form ATP. In the laboratory, the reaction between glucose and oxygen is a small fire. There is no furnace in a living cell. The reaction in a cell is controlled, and it proceeds by a series of steps. An enzyme makes contact with a glucose molecule. The glucose molecule yields four hydrogen atoms. The bond breaks between two carbon atoms. The glucose, with six carbon atoms, divides into two molecules of pyruvic acid, each with three carbons. The pyruvic acid is the fuel that provides the week bonds for each of the steps. The pyruvic acid molecule, as such, is lost from view in the second step. It is incorporated in the subsequent compounds, and provides those compounds with their weakest bonds. glucose -----> pyruvic acid (Fig. 3) and (Fig. 4) |
|
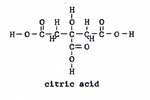
2. pyruvic acid + oxalic acid ------> citric acid + carbon dioxide (Fig. 5) (Fig. 6) |
|
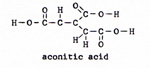
3. citric acid -----> aconitic acid (Fig. 7) |
|
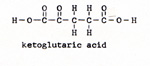
4. aconitic acid ------> ketoglutaric acid + water + carbon dioxide (Fig. 8) |
|
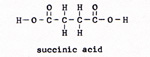
5. ketoglutaric acid ------> succinic acid + water + carbon dioxide (Fig. 9) |
|
6. succinic acid ------> fumaric acid + water (Fig. 10) |
|
7. fumaric acid ------> malic acid (Fig. 11) |
|
malic acid ------> oxalic acid + water Oxalic acid reenters the cycle at step 2. It combines with pyruvic acid. Each cycle consumes one molecule of pyruvic acid. The source of pyruvic acid is glucose. At each step there is another enzyme involved. At several steps ADP is converted into ATP. The waste products are water and carbon dioxide, which are yielded at many steps. This is the citric acid cycle. One molecule of glucose combines with 6 molecules of oxygen and builds 36 molecules of ATP out of ADP and phosphate. A C-C bond is not automatically a weak bond. When the C-C bond is adjacent to two O-C=O groups, it becomes very weak, because the electrons are drawn toward the oxygen atoms. The molecules in the citric acid cycle have many such O-C=O groups. Therefore they have weak C-C bonds and weak C-H bonds. Each molecule is unique. An extra atom or a side chain makes a difference in the reactivity of the molecule. At each step in the cycle, the weakest remaining bonds are broken. Some bacteria never use oxygen in the elemental form. They get energy from glucose. In the absence of oxygen, but with the assistance of enzymes, glucose breaks apart into two molecules of lactic acid. (Fig. 12) |
|
This reaction goes in the right direction. It is linked to the ADP ---->ATP rection, so that it produces two ATP molecules at the same time. There is much in common among all of the kinds of movement to be found in the animal kingdom, and in some of the microbes. Some of the smaller living things have whiplike organelles, known as flagella, which move by means of a set of fibers that they contain. Among the single-celled animals, there are those that propel themselves by cilia, tiny hairs that swing like oars. In humans, the respiratory passages are lined with cilia to move the mucus in the proper direction. The contraction of human muscles depends on fibers that are somewhat like those found in microbes. |
|
The molecules of the muscle fibers are mainly the proteins myosin and actin. One molecule of myosin is equal in weight to about 5 x 105 hydrogen atoms. One molecule of actin has about the weight of 5 x 104 hydrogen atoms. The myosin molecule is a long thread with a bulb on one end. A bundle of myosin molecules forms a thick filament that has some resemblance to the diagram in (Fig.13) |
|
The actin molecule is globular. The thin filament of actin is a double string of molecules that might look like the diagram in (Fig. 14) |
|
The thin filaments alternate with the thick filaments inside the muscle cell. (Fig. 15) |
|
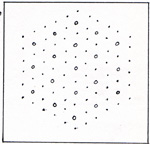
The thin filament looks like a simple line. A cell contains many fibers. A fiber contains many filaments. Figure 15 shows only a small part of one fiber, cut the long way, longitudinal section. A cross section of a small part of one fiber is shown in (Fig. 16) |
|
The circles are the thick filaments. The dots are the thin filaments. In the higher animals, during the embryonic development of a muscle, a chain of several cells combines into a single cell of many nuclei. The muscle fibers thus become continuous over a length that is in the millimeter range. The ends of such chainlike cells are securely glued to adjacent cells. The contraction of a muscle starts with ATP yielding a phosphate group. The phosphate combines with some other kind of molecule. There is an excess of energy from the making of a strong bond. As a result, there is a charge on an actin molecule, and an opposite charge on the head of a myosin molecule. The attractive force between the molecule on the thin filament and the molecule on the thick filament is in the direction that shortens the muscle fiber. Other pairs of molecules also become attracted to each other. The head of each myosin molecule alternately has ATP converting to ADP, and ADP converting to ATP. In the presence of ATP, the charges are no longer separated, and no attraction exists. In the absence of ATP, there is a new point of attraction a step ahead on the actin filament. This causes the fiber to contract still more. It is as if the thick filament were pulling its way along the thin filament, in the manner of a rope climber. A muscle contracts when it gets a signal from the nervous system. The signal is carried by a nerve cell. A nerve cell has a small body amd a long tail, called an axon. The axon of a nerve that extends from the brain to the toe is as long as the nerve. The single axon, as it reaches the muscle, branches out into nearly a hundred nerve endings. In that way, the signal that the nerve carries arrives simultaneously at all of the fibers of that muscle. A signal is transmitted by means of sodium ions, Na+, and potassium ions, K+, that cling to the membrane of the nerve cell. (Fig. 17) |
|
The potassium ions line the inside of the membrane of the axon. The sodium ions coat the outside of the membrane. A signal is a momentary reversal of the ions. The Na+ moves to the inside of the axon, and the K+ moves to the outside. The reversal takes up only a short distance alng the axon. The reversal is like the crest of a wave. It moves along the axon as a pulse. (Fig. 18) |
|
Na+ is represented by o. K+ is represented by *. The signal arrives at the end of the axon. The end of the axon is in contact with the muscle cell. That point in the cell membrane communicates with a system of tubules that are distributed throughout the muscle cell. There are calcium ions, Ca2+ in the tubules. The arrival of a signal causes calcium ions to pass through the membrane of the tubule into the cell plasm. The reactions of ADP and ATP proceed only when Ca2+ ions are present. The Ca2+ ions probably regulate the sequence of action of the myosin heads. Relaxation of a muscle doesn't take place in one jump. If I have an arm raised, I don't just drop it. I let it down according to my conscious control. Muscles also contract according to instructions. A contraction can be mild or strong to any degree, in obedience to nerve impulses. There is an excellent discussion along this line at: Chemistry Laureates |
|
|
| |
|
|
||
| previous page |
|
next page |
|
|
||
|
|
||