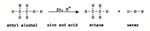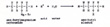Carbon
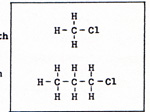
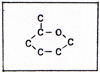
Pure carbon exists mainly in two forms, diamond and graphite. Diamond is one of the hardest substances. Graphite is very soft. Each carbon atom in a diamond is bonded to four others. (Fig. 1) |
|
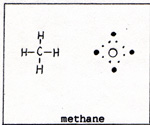
There are no excess outer electrons without bonding sites. In graphite each atom is bonded to three others. There is an outer electron on each atom that is not participating in bonding. If two wires from an electrochemical battery are touched to two points on a piece of graphite, the unbonded electrons shift easily from atom to atom, constituting an electric current. The crystal of graphite is composed of sheets, piled one over the other. (Fig. 1) The softness of graphite is due to the slippage of the layers past one another. A diamond can burn in air at 1000 K. Graphite burns in air at kindling temperatures that depend on the size and structure of the piece of graphite. Coal is mainly graphite, but it is hard to ignite. Graphite conducts heat. If heat is applied to one side of a piece of coal, the heat is conducted to the rest of the coal, preventing the ignition of the coal until enough heat accumulates. A wooden match is easily ignited. Before the match is consumed through and through, it turns black. The wood of the match is partly hydrogen, partly carbon and oxygen. The hydrogen burns first, leaving carbon as graphite. The carbon is preserved for the time being, by the lack of sufficient oxygen. After the hydrogen is consumed, oxygen burns the carbon. Carbon has difficulty competing with hydrogen, because each carbon atom in graphite has three bonds. Hydrogen has only one bond. Carbon is found uncombined underground as diamond or coal. The carbon in coal is graphite. In addition to graphite, there are minerals and coal tar in the coal. The graphite is not chemically combined with the minerals and tar, but the mixture is intimate. Heating the coal in the absence of fresh air, separates the coal tar from the graphite and minerals. The coal tar is the source of valuable compounds. The coal with the coal tar removed is coke. The burning of coke uses up the graphite, and leaves a residue of mineral ash. Pure carbon can be obtained by situating a metal surface in the flame of burning coke. The metal is cooled by a circulating coolant. For example, the metal may consist of pipes which carry water or steam or some other fluid. In the flame are bits of unburned graphite that have become separated from the coke, but have not yet had contact with sufficient oxygen, The bits of graphite settle on the metal, where they cannot burn because they are kept below kindling temperature. The carbon atoms, being bonded to each other, cannot combine with oxygen at that temperature. Carbon is different from all other elements in the variety of ways in which carbon atoms combine with other carbon atoms, and in its use of double and triple bonds. No other element, except hydrogen, appears in as many compounds as carbon. A compound of carbon and hydrogen is methane, (Fig. 2). |
|
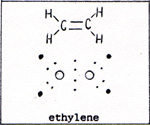
A double bonded compound is ethylene, (Fig. 3) |
|
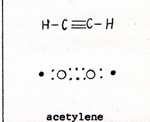
A triple bonded compound is acetylene, (Fig. 4) |
|
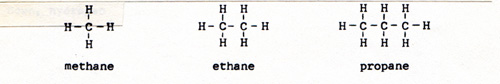
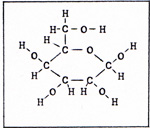
The carbon atom has 6 protons, 6 neutrons, and 6 electrons. Two of the 6 electrons are close to the nucleus, and don't participate in bonding. There are four outer electrons. Carbon can be bonded to four other atoms. There are compounds of carbon and hydrogen of all sizes and shapes. (Fig. 5). |
|
Carbon combines with most of the elements. (Fig. 6) |
|
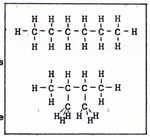
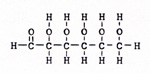
Carbon compounds behave according to the shape and size of the molecule, and according to the elements or groups of elements that are attached to the molecule. The compounds that contain only hydrogen and carbon, and only single bonds, are alkanes. The alkanes are not active. They can be broken apart by heat. They can react with oxygen, fluorine, chlorine, and bromine. Other substances usually don't react with alkanes. If I put a quantity of graphite in a reacting vessel full of hydrogen, I get no reaction. If I heat the reactants, I get a very small yield of compounds like methane, ethane, and propane - more methane than ethane, and more ethane than propane. (Fig. 7) |
|
The reaction goes in the right direction: C + 2H2 -----> CH4 The difficulty is that the strongest bond is the H-H bond. Heat applied to propane breaks the molecule down to ethane, methane, and hydrogen. Hydrogen atoms have to find their way to carbon atoms and to partially formed alkane molecules, faster than the molecules break apart. With the proper catalyst, and the right pressure, the yield of methane is much improved. If we had to, we could produce organic compounds in this way, from the elements. There is a trend in the series - carbon, nitrogen, oxygen, fluorine. The number of protons per atom are: carbon 6, nitrogen 7, oxygen 8, fluorine 9. The more protons there are in the nucleus, the stronger the atom attracts an additional electron. Carbon has to be forced to capture a hydrogen atom. Nitrogen has to be coaxed to capture a hydrogen atom. Oxygen captures hydrogen atoms at kindling temperature. Fluorine captures hydrogen atoms spontaneously at room temperature. In order to produce an alkane, I start with an alcohol, a strong acid, and zinc : (Fig. 8) |
|
When an alkane reacts with oxygen or fluorine, it goes up in flames. The only moderate and controlled reactions of alkanes are with chlorine or bromine. Carbon does not attract electrons strongly. Chlorine and bromine do attract electrons strongly, but not with the extreme strength of oxygen and fluorine. Chlorine attracts the electron of the hydrogen atom and pulls hydrogen away from carbon. (Fig. 9) |
|
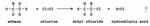
Alkanes with fewer carbon atoms, because of their lighter weight, evaporate at lower temperatures. A second reason why a larger molecule has a higher boiling point than a small molecule, is that there is an attraction between molecules that are near each other. The molecules orient themselves so that the positive points of one molecule are close to the negative points of the other molecule. The larger the molecule, the more points of attraction. Some alkanes have all of the carbon atoms in one line. Other alkanes with the same number of carbon atoms are branched. (Fig. 10) |
|
The branched molecule has less linear surface than the straight molecule. There is less surface for attraction between molecules. Therefore the branched molecule has a lower boiling point than the straight molecule. A carbon compound with one atom of chlorine, has some properties in common with other carbon compounds with different numbers of carbon atoms that have one atom of chlorine. (Fig. 11) |
|
The same could be said for compounds with a double bonded oxygen attached. Similarly there are molecules with a hydroxyl group attached, or a nitrogen atom, or a cyanide group, or an amide group. Carbon chemistry is not as simple as reactions in which all bonds are broken and the atoms scrambled, and the products come out right. Carbon compounds have to have the right atoms in the right arrangements. Most bonds must remain undisturbed in the course of a reaction. Suppose I have in mind two particular compounds of two carbons each, which I want to combine into a product of four carbons. I mix ethyl alcohol and acetic acid and get ethyl acetate: (Fig. 12) |
|
That is easy. The four carbon atoms in ethyl acetate are separated by an oxygen atom. I want, instead, to produce a chemical compound with a continuous chain of carbon atoms. I start with two molecules of ethyl alcohol. I react one moecule with hydrogen bromide and magnesium: (Fig. 13) |
|
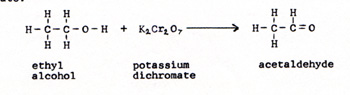
I react the other molecule of ethyl alcohol with potassium dichromate: (Fig. 14) |
|
Ethylmagnesium bromide reacts with acetaldehyde to produce sec-butylmagnesium oxybromide: (Fig. 15) |
|
That is the unbroken chain of four carbons. I put the sec-butylmagnesium oxybromide into an acid solution: (Fig. 16) |
|
I can produce every organic compound if I start with ethyl alcohol. The most complicated molecules have been produced in the laboratory. Usually the synthesis of an organic chemical requires a lot of labor, and the burning of a lot of fuel. For example, sugar can be made in the laboratory, at great expense, from ethyl alcohol. Sugar is made very cheaply by fruit trees, maple trees, vegetables, and, especially, sugar cane. Alcohol is made cheaply by microbes, when they consume sugar. Plants and some microbes start with carbon dioxide and water (plus some minerals) and produce every organic compound that they need to build their own bodies, and to function as living organisms. One of the nicest things about plants is that they yield oxygen to the atmosphere. Otherwise, animals could not exist. To convert carbon dioxide and water into organic molecules requires the breaking of strong bonds, C=O, and O-H, and the forming of weak bonds, C-H, and C-O . Plants use the energy that comes from the sun as photons, to drive the reactions in the direction away from strong bonds toward weak bonds. This is just as important as the recycling of oxygen. Since all chemical reactions break weak bonds and make strong bonds, without the plants that employ solar energy, the quantity of living material would be severely limited. Green plants, bacteria of some kinds, and some protozoa, have, within their cells, organelles called plastids. Some plastids contain chlorophyll for the process of photosynthesis, the production of sugar with the energy of sunlight. Besides chlorophyll, there are, in the plastids, carotenoids and other pigments that absorb light. All colors are absorbed except green. The green photons are scattered, giving leaves their green appearance. Within the plastids are membranes for separating materials and directing the flow of materials. Photons of visible light do not have enough energy to break bonds in H-O-H and O=C=O. The system used by the plant accumulates the energy of several photons and concentrates the energy in bondbreaking. There are several enzymes that direct the reactions in photosynthesis. There is also ATP, adenosine triphosphate, which is present in every living cell of every plant and animal. Energy that is absorbed by chlorophyll is transferred to the reaction that breaks strong bonds to produce ATP, which has weak bonds. Enzymes facilitate the reaction. In several steps, in which ATP is consumed, the bonds are broken in water molecules and carbon dioxide molecules, and sugar, with its weaker bonds, is produced. These reactions are assisted by other enzymes. In short, photons yield energy to form ATP, and ATP reacts to yield energy to form sugar. The details of photosynthesis are still being studied, because they are not completely understood. The overall equation can be written as: (Fig. 17) |
|
Another way to write the formula of glucose is : (Fig. 18) |
|
The molecule is not straight, but hexagonal. (Fig. 19) |
|
A more detailed representation appears in (Fig. 20) |
|
The actual molecule does not lie flat. The four bonds on each carbon atom point outward from the carbon atom as if the carbon atom were in the center of a ping pong ball, and the lines of the bonds were extended to the surface of the ball. The lines would emerge as widely spaced from each other as possible, with an angle of about 109.5o between lines. The flat diagram in figure 20 can be converted into two distinct three-dimensional models, one the mirror image of the other. There are mirror images that are superimposable. I hold my left hand in front of the mirror, palm toward the mirror. I hold my right hand before my eyes, palm toward the eyes, about fifteen inches away. The hands look alike. I try my left glove on each hand. It fits only the left hand. The left hand and its mirror image are not superimposable. I draw the outline of my left hand on one piece of paper, and my right hand on another. They are mirror images. With scissors, I cut the outlines of both images. Now I can't tell which is left and which is right. These mirror images are superimposable. Living things produce or consume only one kind of glucose, D-(+)glucose. The enzymes which produce glucose in plants, can turn out only D-(+)glucose. The enzymes in all living things, which assist in the oxidation of glucose, can operate only on D-(+)glucose. The mirror image of D-(+)glucose is L-(-)glucose. L-(-)glucose is never found in living things. It can be produced in the laboratory. It is not superimposable on D-(+)glucose. The same rule holds for amino acids. Living things use only one form of each amino acid. They can't use the mirror image. If they use the D, they can't use the L of the same compound. If they use the L form of a certain amino acid, they can't use the D form of the same amino acid. |
|
|
| |
|
|
||
| previous page |
|
next page |
|
|
||
|
|
||