Nitrogen
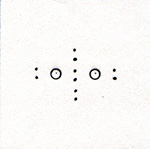
The air is 78% nitrogen. If nitrogen were more reactive, it would not be so abundant in the atmosphere. When compounds that contain nitrogen decompose, they tend to form nitrogen molecules rather than other nitrogen compounds. When two atoms of nitrogen get near each other, the attraction is so strong, that other chemicals can't compete successfully against such a pull. One atom of nitrogen has 7 protons, 7 neutrons, and 7 electrons. The two innermost electrons do not participate in chemical bonding. Of the other five electrons, two are paired, three are single. Electrons on two neighboring atoms of nitrogen are attracted to both atoms. (Fig. 1) Only the outer electrons are shown. |
Fig. 1

|
The atoms are so strongly attracted to each other, that they vibrate with great amplitude. When most of the energy is transferred to the surroundings by collisions with other molecules, the nitogen molecule settles down with the electrons at their points of zero force. (Fig. 2) |
Fig. 2
|
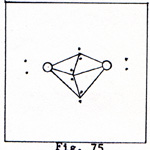
The line of six electrons should be in a three-dimentional array. Figure 3 is an attempt at a rough representation of the nitrogen molecule in three dimensions. Each atom has one unshared pair of electrons. |
Fig. 3
|
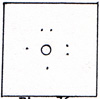
The air is about 79% nitrogen and about 20% oxygen. Oxygen is an active element. There would be no oxygen left in the air if it weren't for the green plants on land and at sea. Oxygen is their waste product. With so much oxygen intermixed with the nitrogen, it is remarkable that the air does not consume itself in one big fire. If I put air into a furnace, and I supply an electric arc, the air does not ignite. Only the molecules of air that are inside the spark react. A very small quantity of nitrogen oxides results. The reactions that form the oxides, require more heat from the spark than they return to the surroundings. Any free nitrogen atoms that appear are more strongly attracted to other nitrogen atoms than to oxygen atoms. The reaction to produce one of the oxides is: N2 + O2 ------> 2NO The product, nitric oxide, is surprising in that it is considered to be triply bonded. The outer electrons of the free oxygen atom are arranged as two pairs and two singles. (Fig. 4) Usually only singles participate in bonding. There should be a maximum of two bonds per oxygen atom. This is another example that illustrates that formal rules have limited scope. |
Fig. 4
|
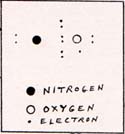
In nitric oxide, three of the electrons of the oxygen atom are in the space between nitrogen and oxygen, and one pair and one single remain with the oxygen atom. (Fig. 5) |
Fig. 5
|
The molecule of nitrogen is inert. The molecule of nitric oxide is chemically active . The oxygen atom has eight protons compared with the nitrogen atom with seven protons. In the nitric oxide molecule, there is an extra single electron on the oxygen atom. The six bonding electrons have their point of zero force closer to the oxygen than to the nitrogen. The nitrogen molecule has no polarity. (Fig. 6) The oxygen end of the nitric oxide molecule is negative relative to the nitrogen end. The polarity of the molecule of NO makes it chemically active. |
Fig. 6

|
The accounting of the bond strengths of the reactants and the products shows that energy from the surroundings must be transferred to the reactants to keep the reaction going. N2 + O2 -----> NO + NO Bond energies are as follows: the triple bond of N2 1.57 x 10-11 erg, the double bond of O2 8.20 x 10-12 erg, and the triple bond of NO 1.04 x 10-11 erg. The total for the reactants is 2.39 x 10-11 erg. The total for the products is 2.08 x 10-11 erg. Subtract 2.08 x 10-11 from 2.39 x 10-11 and get 3.10 x 10-12. The energy of the reaction as written is 3.10 x 10-12 erg. The energy of the reaction to produce one molecule of NO is half of that: 1.55 x 10-12 erg. This is the amount of energy that the reaction receives from the surroundings per NO molecule. When the total bond energies of the reactants is greater than the total bond energies of the products, the transfer of energy is from the surroundings, to the reaction. Any reaction that accepts energy from the environment is more likely to be going in the reverse direction. At a very high temperature there is a reaction between two molecules of nitric oxide that produces nitrogen and oxygen. NO + NO -----> N2 + O2 That reaction yields energy to the environment. When reactants and products can change roles, the reaction is reversible. When the reaction stops or gives the appearance of having stopped, the product of the dominant reaction is present in greater quantity than the product of the reverse reaction. Usually, the dominant reaction is the one that yields energy to the environment. In a gasoline engine, the fuel reacts with oxygen. Instead of admitting pure oxygen into the cylinder of the motor, the engine admits a mixture of air and fuel. As a result, some of the nitrogen of the air combines with some of the oxygen of the air and forms a minute quantity of nitric oxide in the high temperature of the cylinder. This escapes in the exhaust. With hundreds of thousands of cars in a metropolitan area, the quantity of NO in the air becomes significant, and it contributes to the smog. As a molecule in the atmosphere, nitrogen is quite inert at 298 K. Nitrogen reacts with nothing else at room temperature. There is one exception. Lithium metal reacts with nitrogen slowly at room temperature. Lithium can do this because it has a very weak hold on its outer electron, and it has very little mass, so it is easily accelerated. When a nitrogen molecule is close to lithium metal, it is strongly attracted by the electrons of the metal. The electrons, being free to move in the metal, gather where they are attracted by the molecule of nitrogen. The accelerated nitrogen molecule interacts strongly with the metal surface. This increases the vibration of the two nitrogen nuclei. Loosely held electrons leave the lithium to join the nitrogen. The nitrogen atoms that are held together by the electrons that they share, lose their grip on each other when other electrons are available. The combination of vibration and extra electrons causes some increase in the spacing of the two nitrogen atoms. Positive lithium ions are also attracted to the extra electrons. The Li+ ions leave the metal easily because they are weakly held, and easily accelerated. The presence of positive charges helps to weaken the attraction between nitrogen atoms. The atoms separate. Both nitrogen atoms combine with lithium atoms to form lithium nitride. 6Li + N2 ----> 2Li3N In this compound, the extra electrons are closer to nitrogen than to the lithium. The nitrogen becomes a negative ion, N3- and the lithium becomes a positive ion, Li+. The compound takes the form of a crystal. Lithium is the only substance that reacts with nitogen at room temperature, because it is the metal with the lightest atoms. Several others react at high temperatures. There are nitrides of magnesium, calcium, borium, strontium, zinc, cadmium, and thorium. Whenever a nitrogen atom in any compound gets an opportunity to combine with another nitrogen atom, it breaks away to form the nitrogen molecule. In some compounds, existing bonds prevent the ecape of the nitrogen atom. The great attraction of nitrogen for nitrogen is in the form of the triple bond. Single bonds and double bonds between nitrogen atoms exist in some compounds in which the nitrogen atoms cannot break away to form the triple bond. Compare the bond energies of the single, double, and triple bonds of nitrogen to nitrogen.
The bond energies are in the ratio 1 : 2.6 : 5.8 . When there is a N-N bond, each atom is also bonded to two other atoms, as in: H2NNH2. This molecule is short-lived because of the weakness of the N-N bond. Another possibility is : O=N-N=O, also terribly unstable. The kind of attachments that the N-N atoms have to other atoms and molecules changes the strength of the N-N bond. The N-N bond is slightly stronger than 2.70 x 10-12 erg in hydrazobenzene: |
Fig. 7
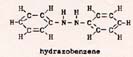
|
The compound azobenzene has doubly bonded nitrogen, and is fairly stable: |
Fig. 8
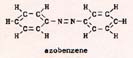
|
When one pair of hydrogen atoms combine, they accelerate toward each other so strongly that they set up a vibration that constitutes a very high temperature. The excess energy is transferred in collisions with other atoms. The commotion may liberate other nitrogen atoms and provoke a chain reaction. After the explosion, one of the products is nitrogen gas. An example of an explosion that is driven by the tendency of nitrogen atoms to combine as nitrogen gas is the decomposition of glyceryl trinitrate, the active ingredient in dynamite. The structural formula of glyceryl trinitrate is : |
Fig. 9
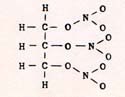
|
The molecule contains 9 N-O bonds. They are very weak bonds, requiring 3.33 x 10-12 erg to break one bond. When one pair of nitrogen atoms combine, they yield 1.57 x 10-11 erg to form one molecule. 1.57 x 10-11 is 4.7 times 3.33 x 10-12. The making of one nitrogen molecule yields enough energy to break almost 5 N-O bonds. The breaking of 5 N-O bonds supplies 5 free atoms of oxygen . When one pair of oxygen atoms combine to form an oxygen molecule, they yield 8.2 x 10-12 erg. 8.2 x 10-12 is 2.46 times 3.33 x 10-12. The making of one oxygen molecule breaks more than 2 N-O bonds. The original 5 free oxygen atoms form at least 2 oxygen molecules. Therefore they break 4 N-O bonds. The total of 5 plus 4 N-O bonds broken releases all of the nitrogen atoms and 6 of the oygen atoms of the glyceryl trinitrate molecule. It is obvious that the energy of one triple bond of nitrogen is enough to start a chain reaction that explodes any quantity of glyceryl trinitrate. Even a small quantity consists of a tremendous number of molecules. To show the proportion of reactant molecules to product molecule, I use a balanced equation: 4C3H5O9N3 -----> 12CO2 + 6N2 + 10 H2O + O2 I have to use 4 molecules of glyceryl trinitrate to get enough atoms to form one molecule of oxygen. It seems remarkable that the products come out in the right proportions. This is a reaction in which all bonds are broken at the same time. Suddenly there is a tremendously high temperature. The only bonds that can exist at that temperature are the nitrogen triple bond and the carbon monoxide triple bond. As the energy transfers to the surroundings, the temperature falls gradually. Bonds can form only when the temperature is low enough. The strongest bonds form first. The rest of the bonds take their turns in order of decreasing strength as the temperature continues to fall. The order is N2 , CO2 , H2O , O2 . Even before N2 forms, CO forms. CO is carbon monoxide. The triple bond of carbon monoxide is the strongest bond in any molecule of two atoms, 1.78 x 10-11 erg, as compared with the 1.57 x 10-11 erg of the nitrogen triple bond. After N2 forms, oxygen atoms react with CO molecules to form CO2. Two double bonds, O=C=O, with a total of 2.48 x 10-11 erg, yield more energy than one triple bond, CO, with 1.78 x 10-11 erg Water forms before oxygen molecules because the two O-H bonds in water yield more energy than the double bond in O=O. Picture a mixture of free atoms of hydrogen and oxygen. When two oxygen atoms are close to each other, they accelerate strongly according to their bond strength of 8.2 x 10-12 erg. As long as the vibrating O=O system retains its energy, it cannot form a bond. As some of the energy is transferred to the surroundings, the vibration has less amplitude and the O=O bond is somewhat established. In the meantime, some hydrogen atoms vibrate with some oxygen atoms. As long as their energy is greater than the bond strength of 8.1 x 10-12 erg, the H-O bonds can't be established. Should a second hydrogen atom start to vibrate with the same oxygen atom, the total vibration in the H-O-H system can be twice 8.1 x 10-12 erg, or 16.2 x 10-12 erg. That doesn't raise the temperature because it is internal energy, nol translational energy. However it tends to tie down an oxygen atom where it is unavailable to other oxygen atoms. It also stabilizes the H-O-H system, because it is harder to increase the total energy of two H-O vibrations than one H-O vibration. Meanwhile, the O=O system can be broken by a slight addition of energy. The free oxygen atoms may find hydrogen atoms to vibrate with, instead of returning to oxygen atoms. On the other hand, knocking one hydrogen atom off H-O-H does not liberate any oxygen atom. Therefore H2O forms before O2. By the same reasoning, ammonia should form before N2 . There is a possible reaction: N2 + 3H2 -----> 2NH3, Nitrogen plus 3Hydrogen yields 2 ammonia. The reaction dosen't take place in this instance, because of the much greater likelihood that two nitrogen atoms find each other than that three successive hydrogen atoms find the same nitrogen atom before a second nitrogen atom arrives and disrupts the process. Whereas H-O-H has more total bond energy than O=O, H-N-H does not have as much total bond energy as triple-bonded nitrogen. The total bond energy of three N-H bonds is slightly more than the bond energy of triple-bonded nitrogen. Therefore, the reaction N2 + 3H2 -----> 2NH3 should take place. The problem is that the reaction rate is so slow, that no noticeable product appears. The problem is solved by the use of a catalyst. A catalyst is anything that speeds up a reaction without yielding energy of its own. For this reaction, the catalyst is iron in a spongy structure that has a large surface area. Possibly, the molecules of reactants are attracted to the surface, and gain kinetic energy that way. There may be somethoing else that the iron does also. Otherwise iron would not be better than some other metal. Even with the catalyst, the production of ammonia is very slow. Merely raising the temperature doesn't help, because the nitrogen and hydrogen occupy more volume at higher temperatures. The space between molecules increases, and the nitrogen and hydrogen molecules can't find each other. To make matters worse, ammonia molecules continue to decompose because they don't have to find anything. They break apart when they interact with anything that supplies the bond energy. The problem is solved by running the reaction at very high pressure. The nitrogen and hydrogen molecules find each other more often when they are densely packed. Meanwhile the decomposition of ammonia does not speed up. Another important contrivance for increasing the yield, removes ammonia selectively. The reaction chamber is a pipe, through which the reactants circulate . At one point in the circuit, the pipe passes through a refrigerated region, where the temperatue is below the boiling point of ammonia, 239.8 K. The ammonia condenses and liquid ammonia is tapped off from the lowest point in the pipe. Removing the product increases the yield, because the product, when removed, does not interfere with the reactant. Furthermore ammonia, which decomposes at high temperature in the reacting vessel, is stable at room temperature. The value of ammonia is its convenience as a starting material in the production of compounds that contain nitrogen. Before the availability of ammonia, chemists had to rely on living things for sources of nitrogen, free of the formidable triple bond. Ammonia is an industrial source of nitric acid: 4NH3 + 5O2 -----> 4NO + 6H2O ammonia + oxygen -----> nitric oxide + water This reaction is carried out at 1100 K, using platinum as a catalyst. After NO is formed, it continues to react with excess oxygen: 2NO + O2 -----> 2NO2 nitric oxide + oxygen -----> nitrogen dioxide The product is cooled to room temperature and dissolved in water: 3NO2 + H2O -----> 2HNO3 + NO nitrogen dioxide + water -----> nitric acid + nitric oxide The nitric oxide is recycled. The nitric acid is sometimes used to produce ammonium nitrate, NH4NO3 , for use as a fertilizer in agriculture. Ammonium nitrate has been known to explode at times. Among the amines are many that can be produced by the reaction of ammonia with an organic compound. For example, the production of methylamine from methyl alcohol, methanol: NH3 + CH3OH -----> CH3NH2 ammonia + methanol -----> methylamine With the help of ammonia, many reactions are facilitated. Furthermore, there are many compounds in which nitrogen is an essential component, amines, amides, azo compounds, and many salts. In living things, proteins, the bulding blocks of the cells, all contain nitrogen. Compounds that are assembled in living cells, are hard to produce in the laboratory, because , in addition to containing the elements in the correct proportions, the molecules of organic compounds have an exact configuration. Each atom must be in a particular place in the molecule. The first organic compound synthesized in the laboratory from inorganic materials was urea: |
Fig. 10
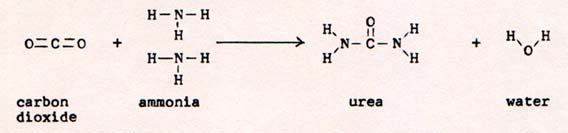
|
An accounting of bond strengths of the reactants and products shows that the reaction is in the right direction. The difficulty is to get gas molecules, CO2 and NH3 to combine correctly to produce solid urea and liquid water. There is a problem of organization. If I were to raise the temperature sufficiently, I could get a mixture of free atoms of carbon, nitrogen, oxygen, and hydrogen, from CO2 and NH3. On cooling, the elements would never combine as urea. I must start with CO2 and NH3 and break no more bonds than necessary. (Fig. 11) |
Fig. 11
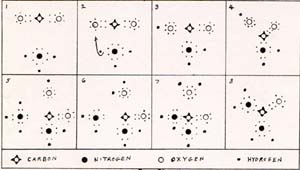
|
1. As a carbon dioxide molecule and an ammonia molecule get close to each other, all of their points of zero force for the electrons and nuclei change, The parts shift in chorus. I can't describe everything at once, although it all happens at once. I show the changes as if they were in sequence. No doubt there is something that leads and something that lags, but I can't tell which leads and which lags.
4. The shifting of negative charge away from the carbon atom leaves it more positive than usual. That attracts the electron pair of the nitrogen atom. A bond forms between the nitrogen and the carbon. 5. A scond molecule of ammonia arrives and orients itself with its nitrogen atom toward the carbon atom. 6. At this point, heat and pressure are applied. One of the protons of the second ammonia molecule is drawn toward the singly bonded oxygen atom. 7. The presence of an extra proton on the oxygen atom draws electrons away from the carbon atom, toward the oxygen atom. 8. Water is removed, and urea remains. The shape of the urea molecule is the result of falling into place of all the parts, at their points of zero force. The accounting of bond energies shows that the reactants have less than 1% higher bond energy than the products. That is why heat and pressure are applied. It also explains why the reaction is reversible. |
|
|
| |
|
|
||
| previous page |
|
next page |
|
|
||
|
|
||