More Chemistry | ||||||||
|
|
|
|
|
|
|
|
|
|
|
|
Hydrogen
I perform an experiment in an empty flask. I put in one electron-proton system and one free electron. The electron of the electron-proton system is at the point of zero force, so this electron-proton system is properly called a hydrogen atom. The free electron is attracted to the hydrogen atom. The hydrogen atom has two sides, the proton side, which is positive, and the electron side, which is negative. The free electron is attracted to the proton side. The electron accelerates as it approaches the hydrogen atom. Eventually, it flies past the point of zero force, and it is repelled by the close-range repulsive force. It is accelerated outward by the repulsive force, and it moves almost to the distance it started with. The point of zero force for an electron-proton-electron system is not so close to the proton as the point of zero force for an electron-proton system. The arrival and departure of the free electron causes the resting electron to change its position twice, once during arrival, once during departure. This leaves the electron oscillating. It has a net gain of charge. The energy of motion that is due to the gain of charge is called kinetic energy.
The source of the charge is within the electron-proton-electron system . At the beginning of the experiment, the proton has 4.803 x 10 -10 esu of pos in addition to a much larger amount of neg-pos. The combined 4.803 x 10-10 esu of the electron and the 4.803 x 10-10 esu of the proton, widely separated, represents a potential energy that would exist between a proton and an electron, if there were no close range repulsive force, and no interfering second electron. The real potential energy between the free electron and the hydrogen atom is 1.2 x 10-12 erg.
Whenever neg and pos exist apart from each other, there is a potential energy that can be converted into kinetic energy, when the neg and pos move toward each other.
In the case of the electron-proton system, the charges can come together only as far as the short-range force permits. Therefore, the only realistic potential energy in the electron-proton system is only about one millionth of the potential energy, 1.6 x 10-6 erg, between the neg of the electron and the pos of the proton. |
|
There are nuclear reactions in which more of the potential energy, 1.64 x 10-6 erg is converted into kinetic energy. Nuclear reactions are not in the category of chemical reactions. As soon as the free electron responds to the pull of the hydrogen atom, it gains kinetic energy at the expense of the 1.2 x 10-12 erg of potential energy of the hydrogen-electron system. The potential energy is equally divided between the hydrogen atom and the free electron. When the electron moves only part of the way, it picks up 2 x 10-13 erg of kinetic energy. The electron pays 1 x 10-13 erg of potential energy. Since the electron is both payer and receiver, and it receives twice as much as it pays, the electron has a net gain of 1 x 10-13 erg for that distance. I don't mention the length of that distance, because it is unknown and immaterial. When the electron reaches its point of zero force, it has its maximum kinetic energy, 1.2 x 10-12 erg. The hydrogen atom without the free electron, is illustrated in (Fig. 1) |
Fig. 1
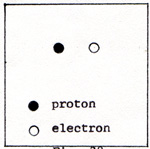 The separation between the proton and the electronn is 5.29 x 10-9 cm.
The separation between the proton and the electronn is 5.29 x 10-9 cm.
When the electron-hydrogen system is at rest, it looks like (Fig. 2)
Fig. 2
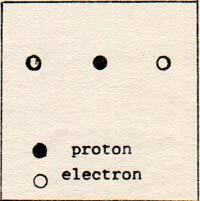 The separation is equal for both electrons; but each electron is more than 5.29 x 10-9 cm away from the proton. The electrons repel each other. The force of repulsion has to be subtracted from the force of attraction, resulting in a new position of zero force.
The separation is equal for both electrons; but each electron is more than 5.29 x 10-9 cm away from the proton. The electrons repel each other. The force of repulsion has to be subtracted from the force of attraction, resulting in a new position of zero force.
|
I return to the free electron, making its first trip in toward the hydrogen atom. As the free electron gets close to the atom, it repels the electron of the hydrogen atom, shifting its point of zero force. This causes the resting electron to accelerate towards its new zero point. Thus charge is added to the formerly resting electron. The force that shifts the electron is the repulsive force between the moving electron and the stationary electron. The force that accelerates one electron, decelerates the other. The incoming electron loses a little speed and a little of its neg-pos charge. The incoming electron rebounds in the short-range repulsive field. It leaves the hydrogen atom with a trifle less velocity than it had on arrival. The outgoing electron stops and turns around before it reaches its original separation from the hydrogen atom. Charge has passed from the free electron to the resting electron. On the second trip, more charge passes to the resting electron. Each trip transfers more charge, until the charges are equal, and both electrons oscillate equally. The electrons may lose their energies of oscillation by interaction with other objects, or by emitting photons. The electron-proton-electron system is also the electron hydrogen system. The official name is "negative hydrogen ion". It has the symbol H-. The hydrogen atom has the symbol H. I experiment with an empty flask that contains a negative hydrogen ion and a proton. The proton is attracted to the ion. I skip the details of oscillations that occur. When the rearrangements are completed, The two electron- two proton system has four points of zero force. (Fig. 3) |
Fig. 3
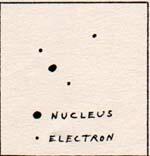
The two-electron-two proton system is known as the hydrogen molecule. A hydrogen molecule consists of two hydrogen atoms. An important concept in chemisty is bond energy, or binding energy. It is the quantity of energy that must be transferred to the system in order to accelerate a part of the system sufficiently to remove the part from the region of attractive force. It is the same quantity of energy as is given up by a part of the system as it arrives from far off, and comes to rest at the point of zero force. Two hydrogen molecules can interact and cause one or more parts to leave a molecule. The most likely part to leave is the one that requires the least energy for removal. That one is the hydrogen atom. For the breaking up of the hydrogen molecule into two hydrogen atoms there is the reaction: H-H -----> H + H . The bond energy is 7.1 x 10-12 erg. The hydrogen atom that leaves is an electron-proton system. The bond between the proton and the electron is intact. If the reaction were: H-H -----> H-H+ + e-, one electron would leave the hydrogen molecule . In order for one electron to leave, 2.3 x 10-11 must be transferred to the electron. 2.3 x 10-11 is more than 3 times 7.1 x 10-12 . The energy of binding one electron to two protons and an electron is three times as great a the binding energy of two hydrogen atoms. Another way to look at this is to watch an electron trying to get away from a hydrogen molecule. It is moving away from two protons. If the electron must leave, one of the protons will feel the repulsion of the other proton at the same time as it feels the attraction of the departing electron. The proton can be expected to depart with the electron. It works the same way for a departing proton. It attracts an electron, which is being repelled by the other electron. The proton can't leave without carrying the electron along with it. In conclusion: if a hydrogen molecule is going to be split, it will divide into two hydrogen atoms. I watch two separate hydrogen atoms. They are attracted to each other. The positive side of one atom attracts the negative side of the other. If the electrons are not vibrating relative to their protons before the interaction, they vibrate after the interaction, because of the shifting of their position of zero force, Some of the kinetic energy of translation of the entire atoms is transferred to the oscillation of the electrons. As the atoms rebound, they do not move away as fast and as far as before the interaction, because of the decrease of kinetic energy of translation. The motions of the atoms continue until the kinetic energy is completely transferred. In the world of our experience, the atmosphere is crowded with molecules, and there are many interactions that remove kinetic energy from pairs of atoms. The average hydrogen molecule in the atmosphere has internal oscillations of all of its parts, and considerable kinetic energy of translation of the molecule as a whole. It is not necessary for the hydrogen-hydrogen system to be completely at rest to be a molecule. Let two hydrogen molecules move toward each other with equal speeds. In their interaction there is a possibility that some energy may transfer from an electron or proton of one molecule to an electron or proton of the other molecule. When that happens, there is a net transfer of energy from one molecule to the other. For the present discussion, I want to concentrate on the motion of the hydrogen molecule as a unit. Therefore I temporarily forbid internal energy. There is, for the moment, only kinetic energy of translation. There is not enough attraction between hydrogen molecules to count in this activity. The interaction of two hydrogen molecules with equal speeds results in no exchange of energy. The molecules rebound with the same energy they had when they arrived. Let two hydrogen molecules move toward each other with unequal speeds. When the molecules are very close to each other, there is a repulsive force which is the sum of the repulsions of electrons for electrons, protons for protons, and the close range repulsive force. The force between any two objects is of equal strength on both objects. Let molecule b have a speed of 20 and molecule c have a speed of 50. The same force that decelerates molecule b to a halt reduces the speed of molecule c to 30. While the force acceleates molecule b from a halt to 20, the same force decelerates moecule c to 10.The force that decelerates molecule c from 10 to zero accelerates molecule b from 20 to 30. Equal forces continue to act between molecule b and molecule c until molecule c has a speed of 20, and molecule b has a speed of 50 in the opposite direction. The final speeds are determined by the strengths of the forces. The initial speeds determine how close the molecules get to each other. The closer the molecules get, the stronger the forces. Evidntly the final speeds are determined by , and are equal to, the initial speeds. Whereas molecule b has an initial speed of 20, and a final speed of 50, molecule c has an initial speed of 50 and a final speed of 20. These collisions are perfect head-on collisions. For collisions that are glancing blows, the motions are analyzed into components, and the same principles are applied as those for the head-on collisions. If molecule b does not meet molecule c exactly head-on, the final velocities are not 50 and 20. Although the total energy for the two molecules that collide remains unchanged, there is a transfer of some energy from one molecule to the other. The rule for molecules of equal mass and equal speed in head-on collision is rebound with no change in speed for either molecule. There is no transfer of energy. For molecules of equal mass and different speeds, the rule is an exchange of speeds. Each molecule receives the direction and speed of the other. Energy is transferred from the molecule that loses speed to the molecule that gains speed. For molecules moving in the same direction, the energy is transferred from the overtaker to the overtaken. The force between the overtaker and the overtaken pushes against the front of the overtaker, decelerating the overtaker, and pushes against the rear of the overtaken, accelerating the overtaken. Hydrogen molecules can collide with helium atoms, but hydrogen and helium do not interact chemically unless extreme measures are taken. Helium is a combination of two protons, two neutrons, and two electrons. The two protons and two neutrons stick together in a tiny space of roughly 10-12 cm. The repulsion of the protons for each other is somehow overcome by the presence of neutrons. Perhaps the neg-pos of the neutron is reoriented in the presence of protons, and the neg of the neutrons faces outward. The 2 neutron-2 proton combination is the nucleus of the helium atom. The atom is complete when it has 2 electrons outside the nucleus. The electrons are tightly held. Therefore the helium atom does not participate in chemical reactions in which an electron or another atom is attracted to the helium atom. Lithium reacts with hydrogen. The nucleus of lithium has three protons and four neutrons. The complete atom also has three electrons. Two electrons are pulled even closer to the nucleus of lithium than the two electrons are to the helium nucleus. The third lithium electron is loosely held in a position where there is room for several more electrons. (Fig. 4)
If a free atom of lithium were to meet a free atom of hydrogen, the outer electron of the lithium atom would be strongly attracted to the hydrogen atom. The lithium and hydrogen atoms would accelerate toward each other. If the other particles were around to accept the kinetic energy, the lithium and the hydrogen would remain together as a molecule of lithium hydide. (Fig. 7)
The lithium hydride molecule that I have just described is imaginary. It can exist only where the universe is empty, except for the one pair of atoms. Real lithium hydride is not a molecule. It is a crystal that consists of equal numbers of lithium ions and negative hydrogen ions.
The lithium atom without the outer electron is a lithium ion, Li+. The hydrogen atom with an extra electron is a negative hydrogen ion, H-. Each H- is surrounded by six Li+. (Fig. 8)
If a free atom is very close to the surface of a piece of lithium metal, it accelerates toward the metal. It is attracted by the outermost electrons. In turn, the electrons are attracted to the hydrogen atom. The space close to the interface between the hydrogen gas and the lithium solid has a higher average kinetic energy per particle than the rest of the space in the room. The source of the kinetic energy is the attractive force between the electrons and the hydrogen atoms. The prevalence of high kinetic energy at the metal's surface causes a few of the lithium atoms to break loose in the presence of the free hydrogen atoms. That accounts for the slow reaction that occurs at room temperature.
The actual production of lithium hydride is carried out at 725oC. At that temperature, there is a considerable increase in the fraction of hydrogen molecules that are broken into free atoms. Lithium melts at 180.5oC. As a liquid at 725oC, the lithium can evaporate some free atons. The lithium hydride that forms at 725oC is a liquid. The melting point of lithium hydride crystals is 688oC.
The reaction is started by heating the reactants to the proper temperature. Once the reaction is proceeding, heat has to be removed to prevent the temperature from rising still more. When the product is cooled to room temperature, and an accounting is made of all the heat that goes into the reaction, and the heat that comes out, it is found that 1.5 x 10-12 erg has to be transferred from the reaction to the surroundings, for each lithium-hdrogen pair that is produced.
If the reaction is carried out in an insulated reaction vessel, the reactants and products reach an equilibrium temperature that is high enough to prevent the formation of any solid lithium or solid lithium hydride. Much of the lithium liquid remains unreacted, and much of it is in the vapor state and unreacted. The lithium hydride, which decomposes completely at 900oC, should be in equilibrium with its components at slghtly less than 900oC. Some lithium hydride forms, and some lithium hydide decomposes. Some hydrogen atoms are free, and most of the hydrogen is in the form of molecules.
 The protons can't get closer to each other, because they repel each other. The protons can't leave, because they are attracted to two electrons. The electrons also repel each other, and remain to be near protons. The electron-electron distance is shorter than the proton-proton distance, because there may be something to the notion of "spin". They say that opposite spins attract. Spin is a misnomer.
The protons can't get closer to each other, because they repel each other. The protons can't leave, because they are attracted to two electrons. The electrons also repel each other, and remain to be near protons. The electron-electron distance is shorter than the proton-proton distance, because there may be something to the notion of "spin". They say that opposite spins attract. Spin is a misnomer.
Fig. 4
 There is an attraction between two lithium atoms. The two combined atoms look like (Fig. 5)
There is an attraction between two lithium atoms. The two combined atoms look like (Fig. 5)
Fig. 5
 There is still ample room for electrons around the group. A third atom joins the first two. (Fig. 6)
There is still ample room for electrons around the group. A third atom joins the first two. (Fig. 6)
Fig. 6
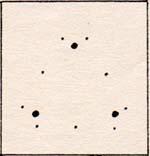 No matter how many lithium atoms are added, there is always an attraction for more atoms. The atoms continue to assemble, until a large piece of solid lithium metal is formed.
No matter how many lithium atoms are added, there is always an attraction for more atoms. The atoms continue to assemble, until a large piece of solid lithium metal is formed.
Fig. 7
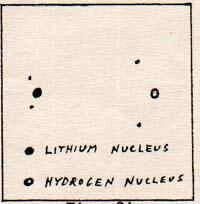 The lithium nucleus cannot get any closer to the hydrogen nucleus, because the positive charges repel each other. The two electrons between the nuclei remain closer to the hydrogen proton than to the lithium nucleus, because the first two electrons of the lthium atom are crowded around the nucleus, where they partially neutralize the positive charge of the nucleus.
The lithium nucleus cannot get any closer to the hydrogen nucleus, because the positive charges repel each other. The two electrons between the nuclei remain closer to the hydrogen proton than to the lithium nucleus, because the first two electrons of the lthium atom are crowded around the nucleus, where they partially neutralize the positive charge of the nucleus.
Fig. 8
 Lithium metal and hydrogen gas , with no other substance present, react imperceptibly at room temperature. The elements can't combine without first becoming free atoms. A tiny fraction of the hydrogen molecules have atoms that vibrate with sufficient amplitude to become separated.
Lithium metal and hydrogen gas , with no other substance present, react imperceptibly at room temperature. The elements can't combine without first becoming free atoms. A tiny fraction of the hydrogen molecules have atoms that vibrate with sufficient amplitude to become separated.
also on chemistry see: | |
|
|
|
|
|
| |
|
|
||
|
|
next page | |
|
|
||
|
|
||